The mechanism of transport by mitochondrial carriers based on analysis of symmetry
- PMID: 19001266
- PMCID: PMC2582046
- DOI: 10.1073/pnas.0809580105
The mechanism of transport by mitochondrial carriers based on analysis of symmetry
Abstract
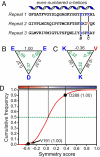
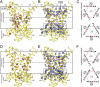
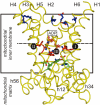
The structures of mitochondrial transporters and uncoupling proteins are 3-fold pseudosymmetrical, but their substrates and coupling ions are not. Thus, deviations from symmetry are to be expected in the substrate and ion-binding sites in the central aqueous cavity. By analyzing the 3-fold pseudosymmetrical repeats from which their sequences are made, conserved asymmetric residues were found to cluster in a region of the central cavity identified previously as the common substrate-binding site. Conserved symmetrical residues required for the transport mechanism were found at the water-membrane interfaces, and they include the three PX[DE]XX[RK] motifs, which form a salt bridge network on the matrix side of the cavity when the substrate-binding site is open to the mitochondrial intermembrane space. Symmetrical residues in three [FY][DE]XX[RK] motifs are on the cytoplasmic side of the cavity and could form a salt bridge network when the substrate-binding site is accessible from the mitochondrial matrix. It is proposed that the opening and closing of the carrier may be coupled to the disruption and formation of the 2 salt bridge networks via a 3-fold rotary twist induced by substrate binding. The interaction energies of the networks allow members of the transporter family to be classified as strict exchangers or uniporters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The transport mechanism of the mitochondrial ADP/ATP carrier.Biochim Biophys Acta. 2016 Oct;1863(10):2379-93. doi: 10.1016/j.bbamcr.2016.03.015. Epub 2016 Mar 19. Biochim Biophys Acta. 2016. PMID: 27001633 Review.
-
Structures of yeast mitochondrial ADP/ATP carriers support a domain-based alternating-access transport mechanism.Proc Natl Acad Sci U S A. 2014 Jan 28;111(4):E426-34. doi: 10.1073/pnas.1320692111. Epub 2014 Jan 13. Proc Natl Acad Sci U S A. 2014. PMID: 24474793 Free PMC article.
-
Structural Mechanism of Transport of Mitochondrial Carriers.Annu Rev Biochem. 2021 Jun 20;90:535-558. doi: 10.1146/annurev-biochem-072820-020508. Epub 2021 Feb 8. Annu Rev Biochem. 2021. PMID: 33556281 Review.
-
The conserved substrate binding site of mitochondrial carriers.Biochim Biophys Acta. 2006 Sep-Oct;1757(9-10):1237-48. doi: 10.1016/j.bbabio.2006.03.021. Epub 2006 Apr 4. Biochim Biophys Acta. 2006. PMID: 16759636 Review.
-
Mitochondrial carriers in the cytoplasmic state have a common substrate binding site.Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):2617-22. doi: 10.1073/pnas.0509994103. Epub 2006 Feb 9. Proc Natl Acad Sci U S A. 2006. PMID: 16469842 Free PMC article.
Cited by
-
A biophysical study on molecular physiology of the uncoupling proteins of the central nervous system.Biosci Rep. 2015 Jun 12;35(4):e00226. doi: 10.1042/BSR20150130. Biosci Rep. 2015. PMID: 26182433 Free PMC article.
-
The effects of cardiolipin on the structural dynamics of the mitochondrial ADP/ATP carrier in its cytosol-open state.J Lipid Res. 2022 Jun;63(6):100227. doi: 10.1016/j.jlr.2022.100227. Epub 2022 May 12. J Lipid Res. 2022. PMID: 35569528 Free PMC article.
-
FA Sliding as the Mechanism for the ANT1-Mediated Fatty Acid Anion Transport in Lipid Bilayers.Int J Mol Sci. 2023 Sep 5;24(18):13701. doi: 10.3390/ijms241813701. Int J Mol Sci. 2023. PMID: 37762012 Free PMC article.
-
Plant Mitochondrial Carriers: Molecular Gatekeepers That Help to Regulate Plant Central Carbon Metabolism.Plants (Basel). 2020 Jan 17;9(1):117. doi: 10.3390/plants9010117. Plants (Basel). 2020. PMID: 31963509 Free PMC article. Review.
-
Insights into the iron-ome and manganese-ome of Δmtm1 Saccharomyces cerevisiae mitochondria.Metallomics. 2013 Jun;5(6):656-72. doi: 10.1039/c3mt00041a. Metallomics. 2013. PMID: 23598994 Free PMC article.
References
-
- Palmieri F. The mitochondrial transporter family (SLC25): Physiological and pathological implications. Pflügers Arch. 2004;447:689–709. - PubMed
-
- Palmieri F, et al. Identification of mitochondrial carriers in Saccharomyces cerevisiae by transport assay of reconstituted recombinant proteins. Biochim Biophys Acta. 2006;1757:1249–1262. - PubMed
-
- Fleury C, et al. Uncoupling protein-2: A novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997;15:269–272. - PubMed
-
- Boss O, et al. Uncoupling protein-3: A new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett. 1997;408:39–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

