Ex vivo characterization of polyclonal memory CD8+ T-cell responses to PRAME-specific peptides in patients with acute lymphoblastic leukemia and acute and chronic myeloid leukemia
- PMID: 18988867
- PMCID: PMC2652370
- DOI: 10.1182/blood-2008-03-144071
Ex vivo characterization of polyclonal memory CD8+ T-cell responses to PRAME-specific peptides in patients with acute lymphoblastic leukemia and acute and chronic myeloid leukemia
Abstract
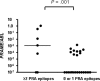
Preferentially expressed antigen of melanoma (PRAME) is aberrantly expressed in hematologic malignancies and may be a useful target for immunotherapy in leukemia. To determine whether PRAME is naturally immunogenic, we studied CD8(+) T-cell responses to 4 HLA-A*0201-restricted PRAME-derived epitopes (PRA100, PRA142, PRA300, PRA425) in HLA-A*0201-positive patients with acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and healthy donors. CD8(+) T cells recognizing PRAME peptides could be detected ex vivo in 4 of 10 ALL, 6 of 10 AML, 3 of 10 CML patients, and 3 of 10 donors by HLA-A2 tetramer analysis and flow cytometry for intracellular interferon-gamma. The frequency of PRAME-specific CD8(+) T cells was greater in patients with AML, CML, and ALL than healthy controls. All peptides were immunogenic in patients, while responses were only detected to PRA300 in donors. High PRAME expression in patient peripheral blood mononuclear cells was associated with responses to greater than or equal to 2 PRAME epitopes compared with low PRAME expression levels (4/7 vs 0/23, P = .001), suggesting a PRAME-driven T-cell response. PRAME-specific T cells were readily expanded in short-term cultures in donors and patients. These results provide evidence for spontaneous T cell reactivity against multiple epitopes of PRAME in ALL, AML, and CML. The potential for developing PRAME as a target for immunotherapy in leukemia deserves further exploration.
Figures




Similar articles
-
Identification and characterization of epitopes of the receptor for hyaluronic acid-mediated motility (RHAMM/CD168) recognized by CD8+ T cells of HLA-A2-positive patients with acute myeloid leukemia.Blood. 2005 Aug 1;106(3):938-45. doi: 10.1182/blood-2004-12-4787. Epub 2005 Apr 12. Blood. 2005. PMID: 15827130
-
Cytotoxic T lymphocytes directed to the preferentially expressed antigen of melanoma (PRAME) target chronic myeloid leukemia.Blood. 2008 Sep 1;112(5):1876-85. doi: 10.1182/blood-2008-04-150045. Epub 2008 Jun 30. Blood. 2008. PMID: 18591381 Free PMC article.
-
Ex vivo characterization of multiepitopic tumor-specific CD8 T cells in patients with chronic myeloid leukemia: implications for vaccine development and adoptive cellular immunotherapy.J Immunol. 2005 Jun 15;174(12):8210-8. doi: 10.4049/jimmunol.174.12.8210. J Immunol. 2005. PMID: 15944330
-
Preferentially expressed antigen of melanoma (PRAME) in the development of diagnostic and therapeutic methods for hematological malignancies.Leuk Lymphoma. 2003 Mar;44(3):439-44. doi: 10.1080/1042819021000035725. Leuk Lymphoma. 2003. PMID: 12688312 Review.
-
Targeting PRAME for acute myeloid leukemia therapy.Front Immunol. 2024 Mar 26;15:1378277. doi: 10.3389/fimmu.2024.1378277. eCollection 2024. Front Immunol. 2024. PMID: 38596687 Free PMC article. Review.
Cited by
-
The Ph-positive and Ph-negative myeloproliferative neoplasms: some topical pre-clinical and clinical issues.Haematologica. 2011 Apr;96(4):590-601. doi: 10.3324/haematol.2010.035675. Epub 2011 Jan 17. Haematologica. 2011. PMID: 21242185 Free PMC article. Review.
-
Lymphodepletion is permissive to the development of spontaneous T-cell responses to the self-antigen PR1 early after allogeneic stem cell transplantation and in patients with acute myeloid leukemia undergoing WT1 peptide vaccination following chemotherapy.Cancer Immunol Immunother. 2012 Jul;61(7):1125-36. doi: 10.1007/s00262-011-1187-z. Epub 2011 Dec 24. Cancer Immunol Immunother. 2012. PMID: 22198310 Free PMC article. Clinical Trial.
-
Outcome of donor-derived TAA-T cell therapy in patients with high-risk or relapsed acute leukemia post allogeneic BMT.Blood Adv. 2022 Apr 26;6(8):2520-2534. doi: 10.1182/bloodadvances.2021006831. Blood Adv. 2022. PMID: 35244681 Free PMC article. Clinical Trial.
-
A Comprehensive Preclinical Model Evaluating the Recombinant PRAME Antigen Combined With the AS15 Immunostimulant to Fight Against PRAME-expressing Tumors.J Immunother. 2015 Oct;38(8):311-20. doi: 10.1097/CJI.0000000000000095. J Immunother. 2015. PMID: 26325375 Free PMC article.
-
Mechanisms of Immune Evasion in Acute Lymphoblastic Leukemia.Cancers (Basel). 2021 Mar 26;13(7):1536. doi: 10.3390/cancers13071536. Cancers (Basel). 2021. PMID: 33810515 Free PMC article. Review.
References
-
- Ikeda H, Lethe B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199–208. - PubMed
-
- van Baren N, Chambost H, Ferrant A, et al. PRAME, a gene encoding an antigen recognized on a human melanoma by cytolytic T cells, is expressed in acute leukaemia cells. Br J Haematol. 1998;102:1376–1379. - PubMed
-
- van't Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. - PubMed
-
- Boon K, Edwards JB, Siu IM, et al. Comparison of medulloblastoma and normal neural transcriptomes identifies a restricted set of activated genes. Oncogene. 2003;22:7687–7694. - PubMed
-
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835–847. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

