Activated STAT3 is a mediator and biomarker of VEGF endothelial activation
- PMID: 18981713
- PMCID: PMC2932444
- DOI: 10.4161/cbt.7.12.6967
Activated STAT3 is a mediator and biomarker of VEGF endothelial activation
Abstract
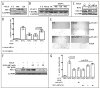
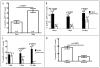
STAT3 plays important roles in cell proliferation and survival signaling and is often constitutively activated in transformed cells. In this study, we examined STAT3 activation in endothelial cells (EC) during angiogenic activation and therapeutic angiogenesis inhibition. VEGF stimulation of cultured EC induced STAT3 phosphorylation by a VEGFR2- and Src-dependent mechanism. FGF2 but not PlGF also induced EC STAT3 activation in vitro. Activated STAT3 mediated VEGF induction of EC Bcl-2 and contributed to VEGF protection of EC from apoptosis. In vivo, p-STAT3 was absent by immunohistological staining in the vascular EC of most normal mouse organs but was present in the vessels of mouse and human tumors. Tumor vascular p-STAT3 increased as tumors were induced to overexpress VEGF, indicating that VEGF is an activator of EC p-STAT3 in vivo. Tumor vascular p-STAT3 decreased during angiogenesis inhibition by antagonists of VEGF-VEGFR signaling, VEGF Trap and SU5416, indicating that VEGF contributed to the EC STAT3 activation seen in the tumors prior to treatment and that p-STAT3 may be used to monitor therapy. These studies show that p-STAT3 is a mediator and biomarker of endothelial activation that reports VEGF-VEGFR2 activity and may be useful for studying the pharmacodynamics of targeted angiogenesis inhibitors.
Figures




Comment in
-
The current STATe of biomarkers to predict the response to anti-angiogenic therapies.Cancer Biol Ther. 2008 Dec;7(12):2004-6. doi: 10.4161/cbt.7.12.7191. Cancer Biol Ther. 2008. PMID: 19158482 No abstract available.
Similar articles
-
Novel role of ARF6 in vascular endothelial growth factor-induced signaling and angiogenesis.Circ Res. 2005 Mar 4;96(4):467-75. doi: 10.1161/01.RES.0000158286.51045.16. Epub 2005 Feb 3. Circ Res. 2005. PMID: 15692085
-
Necl-5/poliovirus receptor interacts with VEGFR2 and regulates VEGF-induced angiogenesis.Circ Res. 2012 Mar 2;110(5):716-26. doi: 10.1161/CIRCRESAHA.111.256834. Epub 2012 Jan 26. Circ Res. 2012. PMID: 22282193
-
Inhibition of STAT3 signaling pathway by nitidine chloride suppressed the angiogenesis and growth of human gastric cancer.Mol Cancer Ther. 2012 Feb;11(2):277-87. doi: 10.1158/1535-7163.MCT-11-0648. Epub 2011 Dec 27. Mol Cancer Ther. 2012. PMID: 22203730
-
STAT3: a critical transcription activator in angiogenesis.Med Res Rev. 2008 Mar;28(2):185-200. doi: 10.1002/med.20101. Med Res Rev. 2008. PMID: 17457812 Review.
-
Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy.Curr Med Chem Anticancer Agents. 2003 Mar;3(2):95-117. doi: 10.2174/1568011033353452. Curr Med Chem Anticancer Agents. 2003. PMID: 12678905 Review.
Cited by
-
Inhibition of the angiogenesis and growth of Aloin in human colorectal cancer in vitro and in vivo.Cancer Cell Int. 2013 Jul 12;13(1):69. doi: 10.1186/1475-2867-13-69. Cancer Cell Int. 2013. PMID: 23848964 Free PMC article.
-
Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells.J Clin Invest. 2010 Feb;120(2):457-71. doi: 10.1172/JCI40483. Epub 2010 Jan 19. J Clin Invest. 2010. PMID: 20093776 Free PMC article.
-
Exosomes Derived From CTF1-Modified Bone Marrow Stem Cells Promote Endometrial Regeneration and Restore Fertility.Front Bioeng Biotechnol. 2022 Apr 13;10:868734. doi: 10.3389/fbioe.2022.868734. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35497344 Free PMC article.
-
Therapeutic Targeting of the Proinflammatory IL-6-JAK/STAT Signalling Pathways Responsible for Vascular Restenosis in Type 2 Diabetes Mellitus.Cardiol Res Pract. 2019 Jan 2;2019:9846312. doi: 10.1155/2019/9846312. eCollection 2019. Cardiol Res Pract. 2019. PMID: 30719343 Free PMC article. Review.
-
Combination BRAFV600E Inhibition with the Multitargeting Tyrosine Kinase Inhibitor Axitinib Shows Additive Anticancer Activity in BRAFV600E-Mutant Anaplastic Thyroid Cancer.Thyroid. 2023 Oct;33(10):1201-1214. doi: 10.1089/thy.2023.0201. Epub 2023 Oct 3. Thyroid. 2023. PMID: 37675898 Free PMC article.
References
-
- Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359–64. - PubMed
-
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. - PubMed
-
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. - PubMed
-
- Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, von Recum HA, Yuan J, Kamihara J, Flynn E, D’Amato R, Folkman J, Mulligan RC. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci USA. 2001;98:4605–10. - PMC - PubMed
-
- Siemeister G, Weindel K, Mohrs K, Barleon B, Martiny-Baron G, Marme D. Reversion of deregulated expression of vascular endothelial growth factor in human renal carcinoma cells by von Hippel-Lindau tumor suppressor protein. Cancer Res. 1996;56:2299–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
