Plasmin activates epithelial Na+ channels by cleaving the gamma subunit
- PMID: 18981180
- PMCID: PMC2605981
- DOI: 10.1074/jbc.M805676200
Plasmin activates epithelial Na+ channels by cleaving the gamma subunit
Abstract
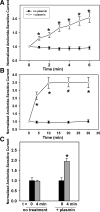
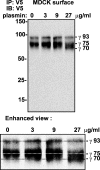
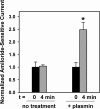
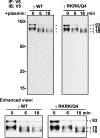
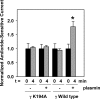
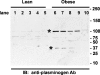
Proteolytic processing of epithelial sodium channel (ENaC) subunits occurs as channels mature within the biosynthetic pathway. The proteolytic processing events of the alpha and gamma subunits are associated with channel activation. Furin cleaves the alpha subunit ectodomain at two sites, releasing an inhibitory tract and activating the channel. However, furin cleaves the gamma subunit ectodomain only once. A second distal cleavage in the gamma subunit induced by other proteases, such as prostasin and elastase, is required to release a second inhibitory tract and further activate the channel. We found that the serine protease plasmin activates ENaC in association with inducing cleavage of the gamma subunit at gammaLys194, a site distal to the furin site. A gammaK194A mutant prevented both plasmin-dependent activation of ENaC and plasmin-dependent production of a unique 70-kDa carboxyl-terminal gamma subunit cleavage fragment. Plasmin-dependent cleavage and activation of ENaC may have a role in extracellular volume expansion in human disorders associated with proteinuria, as filtered plasminogen may be processed by urokinase, released from renal tubular epithelium, to generate active plasmin.
Figures
Similar articles
-
TMPRSS4-dependent activation of the epithelial sodium channel requires cleavage of the γ-subunit distal to the furin cleavage site.Am J Physiol Renal Physiol. 2012 Jan 1;302(1):F1-8. doi: 10.1152/ajprenal.00330.2011. Epub 2011 Oct 12. Am J Physiol Renal Physiol. 2012. PMID: 21993886 Free PMC article.
-
Prostasin interacts with the epithelial Na+ channel and facilitates cleavage of the γ-subunit by a second protease.Am J Physiol Renal Physiol. 2014 Nov 1;307(9):F1080-7. doi: 10.1152/ajprenal.00157.2014. Epub 2014 Sep 10. Am J Physiol Renal Physiol. 2014. PMID: 25209858 Free PMC article.
-
Plasmin and chymotrypsin have distinct preferences for channel activating cleavage sites in the γ subunit of the human epithelial sodium channel.J Gen Physiol. 2012 Oct;140(4):375-89. doi: 10.1085/jgp.201110763. Epub 2012 Sep 10. J Gen Physiol. 2012. PMID: 22966015 Free PMC article.
-
New role for plasmin in sodium homeostasis.Curr Opin Nephrol Hypertens. 2010 Jan;19(1):13-9. doi: 10.1097/MNH.0b013e3283330fb2. Curr Opin Nephrol Hypertens. 2010. PMID: 19864949 Free PMC article. Review.
-
Physiological regulation of epithelial sodium channel by proteolysis.Curr Opin Nephrol Hypertens. 2011 Sep;20(5):529-33. doi: 10.1097/MNH.0b013e328348bcc7. Curr Opin Nephrol Hypertens. 2011. PMID: 21670672 Review.
Cited by
-
The epithelial sodium channel (ENaC) establishes a trafficking vesicle pool responsible for its regulation.PLoS One. 2012;7(9):e46593. doi: 10.1371/journal.pone.0046593. Epub 2012 Sep 28. PLoS One. 2012. PMID: 23029554 Free PMC article.
-
The Role of the Plasminogen/Plasmin System in Inflammation of the Oral Cavity.Cells. 2023 Jan 30;12(3):445. doi: 10.3390/cells12030445. Cells. 2023. PMID: 36766787 Free PMC article. Review.
-
Defining an inhibitory domain in the gamma subunit of the epithelial sodium channel.Am J Physiol Renal Physiol. 2010 Oct;299(4):F854-61. doi: 10.1152/ajprenal.00316.2010. Epub 2010 Jul 14. Am J Physiol Renal Physiol. 2010. PMID: 20630937 Free PMC article.
-
Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC).Pflugers Arch. 2009 May;458(1):111-35. doi: 10.1007/s00424-009-0656-0. Epub 2009 Mar 11. Pflugers Arch. 2009. PMID: 19277701 Review.
-
A variant of ASIC2 mediates sodium retention in nephrotic syndrome.JCI Insight. 2021 Aug 9;6(15):e148588. doi: 10.1172/jci.insight.148588. JCI Insight. 2021. PMID: 34166227 Free PMC article.
References
-
- Sheng, S., Johnson, J. P., and Kleyman, T. R. (2008) in The Kidney, Physiology and Pathophysiology (Alpern, R. J., and Hebert, S. C., eds) 4th Ed., pp. 743-68 Elsevier Publishing, Burlington, MA
-
- Kleyman, T. R., Myerburg, M. M., and Hughey, R. P. (2006) Kidney Int. 70 1391-1392 - PubMed
-
- Hughey, R. P., Carattino, M. D., and Kleyman, T. R. (2007) Curr. Opin. Nephrol. Hypertens. 16 444-450 - PubMed
-
- Hughey, R. P., Bruns, J. B., Kinlough, C. L., Harkleroad, K. L., Tong, Q., Carattino, M. D., Johnson, J. P., Stockand, J. D., and Kleyman, T. R. (2004) J. Biol. Chem. 279 18111-18114 - PubMed
-
- Bruns, J. B., Carattino, M. D., Sheng, S., Maarouf, A. B., Weisz, O. A., Pilewski, J. M., Hughey, R. P., and Kleyman, T. R. (2007) J. Biol. Chem. 282 6153-6160 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

