A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy
- PMID: 18951617
- PMCID: PMC3764488
- DOI: 10.1016/j.jaci.2008.09.030
A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy
Abstract
Background: Orally administered, food-specific immunotherapy appears effective in desensitizing and potentially permanently tolerizing allergic individuals.
Objective: We sought to determine whether milk oral immunotherapy (OIT) is safe and efficacious in desensitizing children with cow's milk allergy.
Methods: Twenty children were randomized to milk or placebo OIT (2:1 ratio). Dosing included 3 phases: the build-up day (initial dose, 0.4 mg of milk protein; final dose, 50 mg), daily doses with 8 weekly in-office dose increases to a maximum of 500 mg, and continued daily maintenance doses for 3 to 4 months. Double-blind, placebo-controlled food challenges; end-point titration skin prick tests; and milk protein serologic studies were performed before and after OIT.
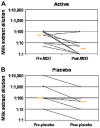
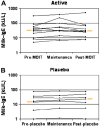
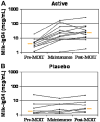
Results: Nineteen patients, 6 to 17 years of age, completed treatment: 12 in the active group and 7 in the placebo group. One dropped out because of persistent eczema during dose escalation. Baseline median milk IgE levels in the active (n = 13) versus placebo (n = 7) groups were 34.8 kUa/L (range, 4.86-314 kUa/L) versus 14.6 kUa/L (range, 0.93-133.4 kUa/L). The median milk threshold dose in both groups was 40 mg at the baseline challenge. After OIT, the median cumulative dose inducing a reaction in the active treatment group was 5140 mg (range 2540-8140 mg), whereas all patients in the placebo group reacted at 40 mg (P = .0003). Among 2437 active OIT doses versus 1193 placebo doses, there were 1107 (45.4%) versus 134 (11.2%) total reactions, with local symptoms being most common. Milk-specific IgE levels did not change significantly in either group. Milk IgG levels increased significantly in the active treatment group, with a predominant milk IgG4 level increase.
Conclusions: Milk OIT appears to be efficacious in the treatment of cow's milk allergy. The side-effect profile appears acceptable but requires further study.
Conflict of interest statement
Disclosure of potential conflict of interest: The rest of the authors have declared that they have no conflict of interest.
Figures

Similar articles
-
The safety and efficacy of sublingual and oral immunotherapy for milk allergy.J Allergy Clin Immunol. 2012 Feb;129(2):448-55, 455.e1-5. doi: 10.1016/j.jaci.2011.10.023. Epub 2011 Nov 30. J Allergy Clin Immunol. 2012. PMID: 22130425 Free PMC article. Clinical Trial.
-
A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow's milk allergy.J Allergy Clin Immunol. 2016 Apr;137(4):1103-1110.e11. doi: 10.1016/j.jaci.2015.10.005. Epub 2015 Nov 12. J Allergy Clin Immunol. 2016. PMID: 26581915 Free PMC article. Clinical Trial.
-
Changes in biomarkers during a six-month oral immunotherapy intervention for cow's milk allergy.Acta Paediatr. 2016 Nov;105(11):1349-1354. doi: 10.1111/apa.13550. Acta Paediatr. 2016. PMID: 27537244 Clinical Trial.
-
Safety and efficacy profile and immunological changes associated with oral immunotherapy for IgE-mediated cow's milk allergy in children: systematic review and meta-analysis.J Investig Allergol Clin Immunol. 2014;24(5):298-307. J Investig Allergol Clin Immunol. 2014. PMID: 25345300 Review.
-
Development of natural tolerance and induced desensitization in cow's milk allergy.Pediatr Allergy Immunol. 2013 Mar;24(2):114-21. doi: 10.1111/pai.12004. Epub 2012 Sep 9. Pediatr Allergy Immunol. 2013. PMID: 22957704 Review.
Cited by
-
World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) guideline update - XIII - Oral immunotherapy for CMA - Systematic review.World Allergy Organ J. 2022 Sep 8;15(9):100682. doi: 10.1016/j.waojou.2022.100682. eCollection 2022 Sep. World Allergy Organ J. 2022. PMID: 36185550 Free PMC article.
-
Oral immunotherapy for treatment of egg allergy in children.N Engl J Med. 2012 Jul 19;367(3):233-43. doi: 10.1056/NEJMoa1200435. N Engl J Med. 2012. PMID: 22808958 Free PMC article. Clinical Trial.
-
New modalities of allergen immunotherapy.Hum Vaccin Immunother. 2018;14(12):2848-2863. doi: 10.1080/21645515.2018.1502126. Epub 2018 Sep 14. Hum Vaccin Immunother. 2018. PMID: 30183485 Free PMC article.
-
Food allergy: separating the science from the mythology.Nat Rev Gastroenterol Hepatol. 2010 Jul;7(7):380-400. doi: 10.1038/nrgastro.2010.80. Nat Rev Gastroenterol Hepatol. 2010. PMID: 20606633 Review.
-
Oral Immunotherapy (OIT): A Personalized Medicine.Medicina (Kaunas). 2019 Oct 13;55(10):684. doi: 10.3390/medicina55100684. Medicina (Kaunas). 2019. PMID: 31614929 Free PMC article. Review.
References
-
- Sampson HA. Food allergy. Part 2: diagnosis and management. J Allergy Clin Immunol. 1999;103:981–9. - PubMed
-
- Sicherer SH, Furlong TJ, Munoz-Furlong A, Burks AW, Sampson HA. A voluntary registry for peanut and tree nut allergy: characteristics of the first 5149 registrants. J Allergy Clin Immunol. 2001;108:128–32. - PubMed
-
- Vander Leek TK, Liu AH, Stefanski K, Blacker B, Bock SA. The natural history of peanut allergy in young children and its association with serum peanut-specific IgE. J Pediatr. 2000;137:749–55. - PubMed
-
- Yu JW, Kagan R, Verreault N, Nicolas N, Joseph L, St Pierre Y, et al. Accidental ingestions in children with peanut allergy. J Allergy Clin Immunol. 2006;118:466–72. - PubMed
-
- Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007;119:1016–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

