Actin dynamics is essential for myosin-based transport of membrane organelles
- PMID: 18951026
- PMCID: PMC2583120
- DOI: 10.1016/j.cub.2008.08.070
Actin dynamics is essential for myosin-based transport of membrane organelles
Abstract
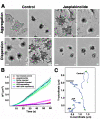
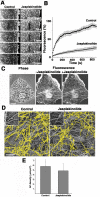
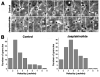
Actin filaments that serve as "rails" for the myosin-based transport of membrane organelles [1-4] continuously turn over by concurrent growth and shortening at the opposite ends [5]. Although it is known that dynamics of actin filaments is essential for many of the actin cytoskeleton functions, the role of such dynamics in myosin-mediated organelle transport was never studied before. Here, we addressed the role of turnover of actin filaments in the myosin-based transport of membrane organelles by treating cells with the drugs that suppress actin-filament dynamics and found that such a suppression significantly inhibited organelle transport along the actin filaments without inhibiting their intracellular distribution or the activity of the myosin motors. We conclude that dynamics of actin filaments is essential for myosin-based transport of membrane organelles and suggest a previously unknown role of actin-filament dynamics in providing the "rails" for continuous organelle movement resulting in the increased distances traveled by membrane organelles along the actin filaments.
Figures

Comment in
-
Organelle transport: dynamic actin tracks for myosin motors.Curr Biol. 2008 Nov 25;18(22):R1066-8. doi: 10.1016/j.cub.2008.09.048. Curr Biol. 2008. PMID: 19036338
Similar articles
-
Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex.J Cell Biol. 1999 Sep 6;146(5):1045-60. doi: 10.1083/jcb.146.5.1045. J Cell Biol. 1999. PMID: 10477758 Free PMC article.
-
Myosin cooperates with microtubule motors during organelle transport in melanophores.Curr Biol. 1998 Jan 29;8(3):161-4. doi: 10.1016/s0960-9822(98)70063-6. Curr Biol. 1998. PMID: 9443916
-
Molecular mechanisms of pigment transport in melanophores.Pigment Cell Res. 1999 Oct;12(5):283-94. doi: 10.1111/j.1600-0749.1999.tb00762.x. Pigment Cell Res. 1999. PMID: 10541038 Review.
-
Interactions and regulation of molecular motors in Xenopus melanophores.J Cell Biol. 2002 Mar 4;156(5):855-65. doi: 10.1083/jcb.200105055. Epub 2002 Feb 25. J Cell Biol. 2002. PMID: 11864991 Free PMC article.
-
Vesicle transport: the role of actin filaments and myosin motors.Microsc Res Tech. 1999 Oct 15;47(2):93-106. doi: 10.1002/(SICI)1097-0029(19991015)47:2<93::AID-JEMT2>3.0.CO;2-P. Microsc Res Tech. 1999. PMID: 10523788 Review.
Cited by
-
PTH-induced internalization of apical membrane NaPi2a: role of actin and myosin VI.Am J Physiol Cell Physiol. 2009 Dec;297(6):C1339-46. doi: 10.1152/ajpcell.00260.2009. Epub 2009 Sep 23. Am J Physiol Cell Physiol. 2009. PMID: 19776390 Free PMC article.
-
Interaction between MyRIP and the actin cytoskeleton regulates Weibel-Palade body trafficking and exocytosis.J Cell Sci. 2016 Feb 1;129(3):592-603. doi: 10.1242/jcs.178285. Epub 2015 Dec 16. J Cell Sci. 2016. PMID: 26675235 Free PMC article.
-
Receptor sorting within endosomal trafficking pathway is facilitated by dynamic actin filaments.PLoS One. 2011;6(5):e19942. doi: 10.1371/journal.pone.0019942. Epub 2011 May 20. PLoS One. 2011. PMID: 21625493 Free PMC article.
-
Activity-Dependence of Synaptic Vesicle Dynamics.J Neurosci. 2017 Nov 1;37(44):10597-10610. doi: 10.1523/JNEUROSCI.0383-17.2017. Epub 2017 Sep 27. J Neurosci. 2017. PMID: 28954868 Free PMC article.
-
Abnormal intermediate filament organization alters mitochondrial motility in giant axonal neuropathy fibroblasts.Mol Biol Cell. 2016 Feb 15;27(4):608-16. doi: 10.1091/mbc.E15-09-0627. Epub 2015 Dec 23. Mol Biol Cell. 2016. PMID: 26700320 Free PMC article.
References
-
- Krendel M, Mooseker MS. Myosins: tails (and heads) of functional diversity. Physiology (Bethesda) 2005;20:239–251. - PubMed
-
- Mooseker MS, Cheney RE. Unconventional myosins. Annu Rev Cell Dev Biol. 1995;11:633–675. - PubMed
-
- Tuxworth RI, Titus MA. Unconventional myosins: anchors in the membrane traffic relay. Traffic. 2000;1:11–18. - PubMed
-
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

