Roles of RIG-I N-terminal tandem CARD and splice variant in TRIM25-mediated antiviral signal transduction
- PMID: 18948594
- PMCID: PMC2575490
- DOI: 10.1073/pnas.0804947105
Roles of RIG-I N-terminal tandem CARD and splice variant in TRIM25-mediated antiviral signal transduction
Abstract
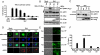
The caspase recruitment domain (CARD) of intracellular adaptors and sensors plays a critical role in the assembly of signaling complexes involved in innate host defense against pathogens and in the regulation of inflammatory responses. The cytosolic receptor retinoic acid-inducible gene-I (RIG-I) recognizes viral RNA in a 5'-triphosphate-dependent manner and initiates an antiviral signaling cascade. Upon viral infection, the N-terminal CARDs of RIG-I undergo the K(63)-linked ubiquitination induced by tripartite motif protein 25 (TRIM25), critical for the interaction of RIG-I with its downstream signaling partner MAVS/VISA/IPS-1/Cardif. Here, we demonstrate the distinct roles of RIG-I first and second CARD in TRIM25-mediated RIG-I ubiquitination: TRIM25 binds the RIG-I first CARD and subsequently ubiquitinates its second CARD. The T(55)I mutation in RIG-I first CARD abolishes TRIM25 interaction, whereas the K(172)R mutation in the second CARD eliminates polyubiquitin attachment. The necessity of the intact tandem CARD for RIG-I function is further evidenced by a RIG-I splice variant (SV) whose expression is robustly up-regulated upon viral infection. The RIG-I SV carries a short deletion (amino acids 36-80) within the first CARD and thereby loses TRIM25 binding, CARD ubiquitination, and downstream signaling ability. Furthermore, because of its robust inhibition of virus-induced RIG-I multimerization and RIG-I-MAVS signaling complex formation, this SV effectively suppresses the RIG-I-mediated IFN-beta production. This study not only elucidates the vital role of the intact tandem CARD for TRIM25-mediated RIG-I activation but also identifies the RIG-I SV as an off-switch regulator of its own signaling pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


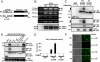

Similar articles
-
Activation of duck RIG-I by TRIM25 is independent of anchored ubiquitin.PLoS One. 2014 Jan 23;9(1):e86968. doi: 10.1371/journal.pone.0086968. eCollection 2014. PLoS One. 2014. PMID: 24466302 Free PMC article.
-
TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity.Nature. 2007 Apr 19;446(7138):916-920. doi: 10.1038/nature05732. Nature. 2007. PMID: 17392790
-
Negative role of RIG-I serine 8 phosphorylation in the regulation of interferon-beta production.J Biol Chem. 2010 Jun 25;285(26):20252-61. doi: 10.1074/jbc.M109.089912. Epub 2010 Apr 20. J Biol Chem. 2010. PMID: 20406818 Free PMC article.
-
Ubiquitin-mediated modulation of the cytoplasmic viral RNA sensor RIG-I.J Biochem. 2012 Jan;151(1):5-11. doi: 10.1093/jb/mvr111. Epub 2011 Sep 2. J Biochem. 2012. PMID: 21890623 Review.
-
TRIM proteins: another class of viral victims.Sci Signal. 2010 Apr 20;3(118):jc2. doi: 10.1126/scisignal.3118jc2. Sci Signal. 2010. PMID: 20407122 Review.
Cited by
-
Ubiquitination in the antiviral immune response.Virology. 2015 May;479-480:52-65. doi: 10.1016/j.virol.2015.02.033. Epub 2015 Mar 7. Virology. 2015. PMID: 25753787 Free PMC article. Review.
-
An Internally Translated MAVS Variant Exposes Its Amino-terminal TRAF-Binding Motifs to Deregulate Interferon Induction.PLoS Pathog. 2015 Jul 29;11(7):e1005060. doi: 10.1371/journal.ppat.1005060. eCollection 2015 Jul. PLoS Pathog. 2015. PMID: 26221961 Free PMC article.
-
Disease classification via gene network integrating modules and pathways.R Soc Open Sci. 2019 Jul 17;6(7):190214. doi: 10.1098/rsos.190214. eCollection 2019 Jul. R Soc Open Sci. 2019. PMID: 31417727 Free PMC article.
-
Functional Evolution of Avian RIG-I-Like Receptors.Genes (Basel). 2018 Sep 12;9(9):456. doi: 10.3390/genes9090456. Genes (Basel). 2018. PMID: 30213147 Free PMC article.
-
The role of ZAP and OAS3/RNAseL pathways in the attenuation of an RNA virus with elevated frequencies of CpG and UpA dinucleotides.Nucleic Acids Res. 2019 Sep 5;47(15):8061-8083. doi: 10.1093/nar/gkz581. Nucleic Acids Res. 2019. PMID: 31276592 Free PMC article.
References
-
- Hiscott J, Lin R, Nakhaei P, Paz S. MasterCARD: A priceless link to innate immunity. Trends Mol Med. 2006;12:53–56. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. - PubMed
-
- Creagh EM, O'Neill LA. TLRs, NLRs and RLRs: A trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–357. - PubMed
-
- Hornung V, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM065367/GM/NIGMS NIH HHS/United States
- RR00168/RR/NCRR NIH HHS/United States
- CA82057/CA/NCI NIH HHS/United States
- CA91819/CA/NCI NIH HHS/United States
- R01 CA082057/CA/NCI NIH HHS/United States
- P51 RR000168/RR/NCRR NIH HHS/United States
- K26 RR000168/RR/NCRR NIH HHS/United States
- CA31363/CA/NCI NIH HHS/United States
- R01 CA091819/CA/NCI NIH HHS/United States
- CA106156/CA/NCI NIH HHS/United States
- R01 CA106156/CA/NCI NIH HHS/United States
- R01 GM065367/GM/NIGMS NIH HHS/United States
- R01 CA031363/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

