Murine retroviruses re-engineered for lineage tracing and expression of toxic genes in the developing chick embryo
- PMID: 18942139
- PMCID: PMC2925429
- DOI: 10.1002/dvdy.21766
Murine retroviruses re-engineered for lineage tracing and expression of toxic genes in the developing chick embryo
Abstract
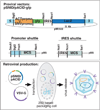
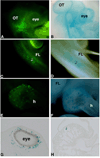
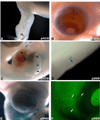
We describe two replication incompetent retroviral vectors that co-express green fluorescent protein (GFP) and beta-galactosidase. These vectors incorporate either the avian reticuloendotheliosis (spleen necrosis virus; SNV) promoter or the chick beta-actin promoter, into the backbone of the murine leukemia (MLV) viral vector. The additional promoters drive transgene expression in avian tissue. The remainder of the vector is MLV-like, allowing high titer viral particle production by means of transient transfection. The SNV promoter produces high and early expression of introduced genes, enabling detection of the single copy integrated GFP gene in infected cells and their progeny in vivo. Substitution of the LacZ coding DNA with a relevant gene of interest will enable its co-expression with GFP, thus allowing visualization of the effect of specific and stable changes in gene expression throughout development. As the VSV-G pseudotyped viral vector is replication incompetent, changes in gene expression can be controlled temporally, by altering the timing of introduction.
Figures


Similar articles
-
Imaging cells in the developing nervous system with retrovirus expressing modified green fluorescent protein.Exp Neurol. 1999 Apr;156(2):394-406. doi: 10.1006/exnr.1999.7033. Exp Neurol. 1999. PMID: 10328944
-
Gene transfer in bovine blastocysts using replication-defective retroviral vectors packaged with Gibbon ape leukemia virus envelopes.Mol Reprod Dev. 1993 Jun;35(2):105-13. doi: 10.1002/mrd.1080350202. Mol Reprod Dev. 1993. PMID: 8391277
-
Retroviral vectors for high-level transgene expression in T lymphocytes.Hum Gene Ther. 2003 Aug 10;14(12):1155-68. doi: 10.1089/104303403322167993. Hum Gene Ther. 2003. PMID: 12908967
-
Transduction of Schistosoma mansoni by vesicular stomatitis virus glycoprotein-pseudotyped Moloney murine leukemia retrovirus.Exp Parasitol. 2006 Apr;112(4):209-20. doi: 10.1016/j.exppara.2006.02.003. Epub 2006 Mar 10. Exp Parasitol. 2006. PMID: 16530185
-
Comparison of the human versus murine cytomegalovirus immediate early gene promoters for transgene expression by adenoviral vectors.J Gen Virol. 1997 Jul;78 ( Pt 7):1653-61. doi: 10.1099/0022-1317-78-7-1653. J Gen Virol. 1997. PMID: 9225042
Cited by
-
Identification of a novel developmental mechanism in the generation of mesothelia.Development. 2012 Aug;139(16):2926-34. doi: 10.1242/dev.082396. Epub 2012 Jul 4. Development. 2012. PMID: 22764055 Free PMC article.
-
Early divergence of central and peripheral neural retina precursors during vertebrate eye development.Dev Dyn. 2015 Mar;244(3):266-76. doi: 10.1002/dvdy.24218. Epub 2014 Nov 17. Dev Dyn. 2015. PMID: 25329498 Free PMC article. Review.
-
Central and peripheral retina arise through distinct developmental paths.PLoS One. 2013 Apr 16;8(4):e61422. doi: 10.1371/journal.pone.0061422. Print 2013. PLoS One. 2013. PMID: 23613848 Free PMC article.
-
Retinal and anterior eye compartments derive from a common progenitor pool in the avian optic cup.Mol Vis. 2011;17:3347-63. Epub 2011 Dec 20. Mol Vis. 2011. PMID: 22219630 Free PMC article.
-
Illumination of neural development by in vivo clonal analysis.Cell Regen. 2018 Oct 12;7(2):33-39. doi: 10.1016/j.cr.2018.09.001. eCollection 2018 Dec. Cell Regen. 2018. PMID: 30671228 Free PMC article. Review.
References
-
- Bell EJ, Brickell PM. Replication-competent retroviral vectors for expressing genes in avian cells in vitro and in vivo. Mol Biotechnol. 1997;7:289–298. - PubMed
-
- Cepko C, Pear W. Overview of the retrovirus transduction system. Chapter 9:Unit9. Curr Protoc Mol Biol. 2001a:9. - PubMed
-
- Cepko C, Pear W. Retrovirus infection of cells in vitro and in vivo. Chapter 9:Unit9. Curr Protoc Mol Biol. 2001b:14. - PubMed
-
- Cepko C, Pear W. Detection of helper virus in retrovirus stocks. Chapter 9:Unit9. Curr Protoc Mol Biol. 2001c:13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

