A ligand peptide motif selected from a cancer patient is a receptor-interacting site within human interleukin-11
- PMID: 18941632
- PMCID: PMC2565473
- DOI: 10.1371/journal.pone.0003452
A ligand peptide motif selected from a cancer patient is a receptor-interacting site within human interleukin-11
Abstract
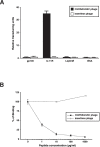
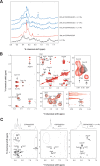
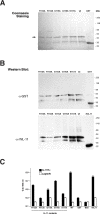
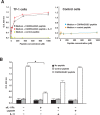
Interleukin-11 (IL-11) is a pleiotropic cytokine approved by the FDA against chemotherapy-induced thrombocytopenia. From a combinatorial selection in a cancer patient, we isolated an IL-11-like peptide mapping to domain I of the IL-11 (sequence CGRRAGGSC). Although this motif has ligand attributes, it is not within the previously characterized interacting sites. Here we design and validate in-tandem binding assays, site-directed mutagenesis and NMR spectroscopy to show (i) the peptide mimics a receptor-binding site within IL-11, (ii) the binding of CGRRAGGSC to the IL-11R alpha is functionally relevant, (iii) Arg4 and Ser8 are the key residues mediating the interaction, and (iv) the IL-11-like motif induces cell proliferation through STAT3 activation. These structural and functional results uncover an as yet unrecognized receptor-binding site in human IL-11. Given that IL-11R alpha has been proposed as a target in human cancer, our results provide clues for the rational design of targeted drugs.
Conflict of interest statement
Figures

Similar articles
-
Modular organization of Interleukin-6 and Interleukin-11 α-receptors.Biochimie. 2015 Dec;119:175-82. doi: 10.1016/j.biochi.2015.11.005. Epub 2015 Nov 10. Biochimie. 2015. PMID: 26551279
-
A designer hyper interleukin 11 (H11) is a biologically active cytokine.BMC Biotechnol. 2012 Mar 21;12:8. doi: 10.1186/1472-6750-12-8. BMC Biotechnol. 2012. PMID: 22433466 Free PMC article.
-
The length of the interleukin-11 receptor stalk determines its capacity for classic signaling.J Biol Chem. 2018 Apr 27;293(17):6398-6409. doi: 10.1074/jbc.RA118.001879. Epub 2018 Mar 9. J Biol Chem. 2018. PMID: 29523682 Free PMC article.
-
The biology of interleukin 11.Stem Cells. 1993 Jul;11 Suppl 2:156-62. doi: 10.1002/stem.5530110825. Stem Cells. 1993. PMID: 8401258 Review.
-
The role of IL-II in hematopoiesis as revealed by a targeted mutation of its receptor.Stem Cells. 1998;16 Suppl 2:53-65. doi: 10.1002/stem.5530160708. Stem Cells. 1998. PMID: 11012177 Review.
Cited by
-
Targeted Phage Display-based Pulmonary Vaccination in Mice and Non-human Primates.Med. 2021 Mar 12;2(3):321-342. doi: 10.1016/j.medj.2020.10.005. Epub 2020 Dec 10. Med. 2021. PMID: 33870243 Free PMC article.
-
Targeted molecular-genetic imaging and ligand-directed therapy in aggressive variant prostate cancer.Proc Natl Acad Sci U S A. 2016 Nov 8;113(45):12786-12791. doi: 10.1073/pnas.1615400113. Epub 2016 Oct 24. Proc Natl Acad Sci U S A. 2016. PMID: 27791181 Free PMC article.
-
Role of the gp85/trans-sialidases in Trypanosoma cruzi tissue tropism: preferential binding of a conserved peptide motif to the vasculature in vivo.PLoS Negl Trop Dis. 2010 Nov 2;4(11):e864. doi: 10.1371/journal.pntd.0000864. PLoS Negl Trop Dis. 2010. PMID: 21072227 Free PMC article.
-
Intracellular targeting of annexin A2 inhibits tumor cell adhesion, migration, and in vivo grafting.Sci Rep. 2017 Jun 26;7(1):4243. doi: 10.1038/s41598-017-03470-w. Sci Rep. 2017. PMID: 28652618 Free PMC article.
-
Discovery of pan-VEGF inhibitory peptides directed to the extracellular ligand-binding domains of the VEGF receptors.Sci Adv. 2016 Oct 28;2(10):e1600611. doi: 10.1126/sciadv.1600611. eCollection 2016 Oct. Sci Adv. 2016. PMID: 27819042 Free PMC article.
References
-
- Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardó-Vila M, et al. Steps toward mapping the human vasculature by phage display. Nat Med. 2002;8:121–127. - PubMed
-
- Zurita AJ, Troncoso P, Cardó-Vila M, Logothetis CJ, Pasqualini R, et al. Combinatorial screenings in patients: the interleukin-11 receptor alpha as a candidate target in the progression of human prostate cancer. Cancer Res. 2004;64:435–439. - PubMed
-
- Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. - PubMed
-
- Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med. 1999;5:1032–1038. - PubMed
-
- Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, et al. Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol. 1999;17:768–774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

