53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility
- PMID: 18931659
- PMCID: PMC2613650
- DOI: 10.1038/nature07433
53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility
Abstract
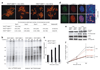
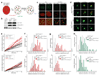
Double-strand breaks activate the ataxia telangiectasia mutated (ATM) kinase, which promotes the accumulation of DNA damage factors in the chromatin surrounding the break. The functional significance of the resulting DNA damage foci is poorly understood. Here we show that 53BP1 (also known as TRP53BP1), a component of DNA damage foci, changes the dynamic behaviour of chromatin to promote DNA repair. We used conditional deletion of the shelterin component TRF2 (also known as TERF2) from mouse cells (TRF2(fl/-)) to deprotect telomeres, which, like double-strand breaks, activate the ATM kinase, accumulate 53BP1 and are processed by non-homologous end joining (NHEJ). Deletion of TRF2 from 53BP1-deficient cells established that NHEJ of dysfunctional telomeres is strongly dependent on the binding of 53BP1 to damaged chromosome ends. To address the mechanism by which 53BP1 promotes NHEJ, we used time-lapse microscopy to measure telomere dynamics before and after their deprotection. Imaging showed that deprotected telomeres are more mobile and sample larger territories within the nucleus. This change in chromatin dynamics was dependent on 53BP1 and ATM but did not require a functional NHEJ pathway. We propose that the binding of 53BP1 near DNA breaks changes the dynamic behaviour of the local chromatin, thereby facilitating NHEJ repair reactions that involve distant sites, including joining of dysfunctional telomeres and AID (also known as AICDA)-induced breaks in immunoglobulin class-switch recombination.
Figures

Similar articles
-
DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion.Nat Cell Biol. 2005 Jul;7(7):712-8. doi: 10.1038/ncb1275. Epub 2005 Jun 19. Nat Cell Biol. 2005. PMID: 15968270
-
Multiple roles for MRE11 at uncapped telomeres.Nature. 2009 Aug 13;460(7257):914-8. doi: 10.1038/nature08196. Epub 2009 Jul 26. Nature. 2009. PMID: 19633651 Free PMC article.
-
DNA-damage response and repair activities at uncapped telomeres depend on RNF8.Nat Cell Biol. 2011 Aug 21;13(9):1139-45. doi: 10.1038/ncb2326. Nat Cell Biol. 2011. PMID: 21857671
-
Double-strand break repair: 53BP1 comes into focus.Nat Rev Mol Cell Biol. 2014 Jan;15(1):7-18. doi: 10.1038/nrm3719. Epub 2013 Dec 11. Nat Rev Mol Cell Biol. 2014. PMID: 24326623 Review.
-
The Connection Between Cell Fate and Telomere.Adv Exp Med Biol. 2021;1275:71-100. doi: 10.1007/978-3-030-49844-3_3. Adv Exp Med Biol. 2021. PMID: 33539012 Review.
Cited by
-
53BP1 mediates productive and mutagenic DNA repair through distinct phosphoprotein interactions.Cell. 2013 Jun 6;153(6):1266-80. doi: 10.1016/j.cell.2013.05.023. Epub 2013 May 30. Cell. 2013. PMID: 23727112 Free PMC article.
-
Transcription regulates telomere dynamics in human cancer cells.RNA. 2012 Apr;18(4):684-93. doi: 10.1261/rna.029587.111. Epub 2012 Feb 22. RNA. 2012. PMID: 22357912 Free PMC article.
-
Impact of histone H4 lysine 20 methylation on 53BP1 responses to chromosomal double strand breaks.PLoS One. 2012;7(11):e49211. doi: 10.1371/journal.pone.0049211. Epub 2012 Nov 28. PLoS One. 2012. PMID: 23209566 Free PMC article.
-
The plant-specific DDR factor SOG1 increases chromatin mobility in response to DNA damage.EMBO Rep. 2022 Dec 6;23(12):e54736. doi: 10.15252/embr.202254736. Epub 2022 Oct 24. EMBO Rep. 2022. PMID: 36278395 Free PMC article.
-
Maintenance of genomic integrity after DNA double strand breaks in the human prostate and seminal vesicle epithelium: the best and the worst.Mol Oncol. 2012 Oct;6(5):473-83. doi: 10.1016/j.molonc.2012.06.001. Epub 2012 Jun 18. Mol Oncol. 2012. PMID: 22762987 Free PMC article. Review.
References
-
- Celli G, de Lange T. DNA processing not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol. 2005;7:712–718. - PubMed
-
- Celli GB, Lazzerini Denchi E, de Lange T. Ku70 stimulates fusion of dysfunctional telomeres yet protects chromosome ends from homologous recombination. Nat Cell Biol. 2006;8:885–890. - PubMed
-
- Lazzerini Denchi E, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448:1068–1071. - PubMed
-
- DiTullio RA, Jr, et al. 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nat Cell Biol. 2002;4:998–1002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY18244/EY/NEI NIH HHS/United States
- OD000379/OD/NIH HHS/United States
- R01 GM049046/GM/NIGMS NIH HHS/United States
- DP1 OD000379-04/OD/NIH HHS/United States
- GM42694/GM/NIGMS NIH HHS/United States
- R37 GM049046/GM/NIGMS NIH HHS/United States
- DP1 OD000379/OD/NIH HHS/United States
- PN2 EY018244/EY/NEI NIH HHS/United States
- Howard Hughes Medical Institute/United States
- GM049046/GM/NIGMS NIH HHS/United States
- R01 GM042694/GM/NIGMS NIH HHS/United States
- R37 GM049046-16/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

