Promotion of lung carcinogenesis by chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model
- PMID: 18927348
- PMCID: PMC2660561
- DOI: 10.1165/rcmb.2008-0198OC
Promotion of lung carcinogenesis by chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model
Abstract
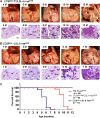
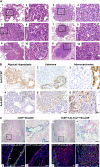
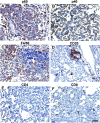
Lung cancer is the leading cause of cancer deaths in the United States. In addition to genetic abnormalities induced by cigarette smoke, several epidemiologic studies have found that smokers with chronic obstructive pulmonary disease (COPD), an inflammatory disease of the lungs, have an increased risk of lung cancer (1.3- to 4.9-fold) compared to smokers without COPD. This suggests a link between chronic airway inflammation and lung carcinogenesis, independent of tobacco smoke exposure. We studied this association by assaying the inflammatory impact of products of nontypeable Haemophilus influenzae, which colonizes the airways of patients with COPD, on lung cancer promotion in mice with an activated K-ras mutation in their airway epithelium. Two new mouse models of lung cancer were generated by crossing mice harboring the LSL-K-ras(G12D) allele with mice containing Cre recombinase inserted into the Clara cell secretory protein (CCSP) locus, with or without the neomycin cassette excised (CCSP(Cre) and CCSP(Cre-Neo), respectively). Lung lesions in CCSP(Cre-Neo)/LSL-K-ras(G12D) and CCSP(Cre)/LSL-K-ras(G12D) mice appeared at 4 and 1 month of age, respectively, and were classified as epithelial hyperplasia of the bronchioles, adenoma, and adenocarcinoma. Weekly exposure of CCSP(Cre)/LSL-K-ras(G12D) mice to aerosolized nontypeable Haemophilus influenzae lysate from age 6-14 weeks resulted in neutrophil/macrophage/CD8 T-cell-associated COPD-like airway inflammation, a 3.2-fold increase in lung surface tumor number (156 +/- 9 versus 45 +/- 7), and an increase in total lung tumor burden. We conclude that COPD-like airway inflammation promotes lung carcinogenesis in a background of a G12D-activated K-ras allele in airway secretory cells.
Figures

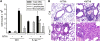
Similar articles
-
Nontypeable Haemophilus influenzae in chronic obstructive pulmonary disease and lung cancer.Int J Chron Obstruct Pulmon Dis. 2011;6:113-23. doi: 10.2147/COPD.S15417. Epub 2011 Jan 27. Int J Chron Obstruct Pulmon Dis. 2011. PMID: 21407824 Free PMC article. Review.
-
Interleukin 6, but not T helper 2 cytokines, promotes lung carcinogenesis.Cancer Prev Res (Phila). 2011 Jan;4(1):51-64. doi: 10.1158/1940-6207.CAPR-10-0180. Epub 2010 Nov 22. Cancer Prev Res (Phila). 2011. PMID: 21098042 Free PMC article.
-
T helper 17 cells play a critical pathogenic role in lung cancer.Proc Natl Acad Sci U S A. 2014 Apr 15;111(15):5664-9. doi: 10.1073/pnas.1319051111. Epub 2014 Mar 31. Proc Natl Acad Sci U S A. 2014. PMID: 24706787 Free PMC article.
-
Toll-like receptors 2, 4, and 9 modulate promoting effect of COPD-like airway inflammation on K-ras-driven lung cancer through activation of the MyD88/NF-ĸB pathway in the airway epithelium.Front Immunol. 2023 May 22;14:1118721. doi: 10.3389/fimmu.2023.1118721. eCollection 2023. Front Immunol. 2023. PMID: 37283745 Free PMC article.
-
Club Cell Protein 16 (CC16) Augmentation: A Potential Disease-modifying Approach for Chronic Obstructive Pulmonary Disease (COPD).Expert Opin Ther Targets. 2016 Jul;20(7):869-83. doi: 10.1517/14728222.2016.1139084. Epub 2016 Feb 11. Expert Opin Ther Targets. 2016. PMID: 26781659 Free PMC article. Review.
Cited by
-
NOS2 enhances KRAS-induced lung carcinogenesis, inflammation and microRNA-21 expression.Int J Cancer. 2013 Jan 1;132(1):9-18. doi: 10.1002/ijc.27644. Epub 2012 Jun 13. Int J Cancer. 2013. PMID: 22618808 Free PMC article.
-
Long-term exposure to house dust mites accelerates lung cancer development in mice.J Exp Clin Cancer Res. 2023 Jan 21;42(1):26. doi: 10.1186/s13046-022-02587-9. J Exp Clin Cancer Res. 2023. PMID: 36670473 Free PMC article.
-
Nontypeable Haemophilus influenzae in chronic obstructive pulmonary disease and lung cancer.Int J Chron Obstruct Pulmon Dis. 2011;6:113-23. doi: 10.2147/COPD.S15417. Epub 2011 Jan 27. Int J Chron Obstruct Pulmon Dis. 2011. PMID: 21407824 Free PMC article. Review.
-
Bronchoalveolar Lavage Fluid Utilized Ex Vivo to Validate In Vivo Findings: Inhibition of Gap Junction Activity in Lung Tumor Promotion is Toll-Like Receptor 4-Dependent.J Mol Biomark Diagn. 2013 Dec 27;5(1):22168. doi: 10.4172/2155-9929.1000160. J Mol Biomark Diagn. 2013. PMID: 25035812 Free PMC article.
-
Enhancement of lung tumorigenesis in a Gprc5a Knockout mouse by chronic extrinsic airway inflammation.Mol Cancer. 2012 Jan 12;11:4. doi: 10.1186/1476-4598-11-4. Mol Cancer. 2012. PMID: 22239913 Free PMC article.
References
-
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin 2005;55:10–30. - PubMed
-
- Stellman SD, Takezaki T, Wang L, Chen Y, Citron ML, Djordjevic MV, Harlap S, Muscat JE, Neugut AI, Wynder EL, et al. Smoking and lung cancer risk in American and Japanese men: an international case-control study. Cancer Epidemiol Biomarkers Prev 2001;10:1193–1199. - PubMed
-
- Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park) 2002;16:217–226, 229; discussion 230–232. - PubMed
-
- Tockman MS, Anthonisen NR, Wright EC, Donithan MG. Airways obstruction and the risk for lung cancer. Ann Intern Med 1987;106:512–518. - PubMed
-
- Skillrud DM, Offord KP, Miller RD. Higher risk of lung cancer in chronic obstructive pulmonary disease: a prospective, matched, controlled study. Ann Intern Med 1986;105:503–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

