Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia
- PMID: 18843297
- PMCID: PMC2569873
- DOI: 10.1038/emboj.2008.206
Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia
Abstract
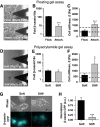
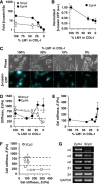
In the mammary gland, epithelial cells are embedded in a 'soft' environment and become functionally differentiated in culture when exposed to a laminin-rich extracellular matrix gel. Here, we define the processes by which mammary epithelial cells integrate biochemical and mechanical extracellular cues to maintain their differentiated phenotype. We used single cells cultured on top of gels in conditions permissive for beta-casein expression using atomic force microscopy to measure the elasticity of the cells and their underlying substrata. We found that maintenance of beta-casein expression required both laminin signalling and a 'soft' extracellular matrix, as is the case in normal tissues in vivo, and biomimetic intracellular elasticity, as is the case in primary mammary epithelial organoids. Conversely, two hallmarks of breast cancer development, stiffening of the extracellular matrix and loss of laminin signalling, led to the loss of beta-casein expression and non-biomimetic intracellular elasticity. Our data indicate that tissue-specific gene expression is controlled by both the tissues' unique biochemical milieu and mechanical properties, processes involved in maintenance of tissue integrity and protection against tumorigenesis.
Figures

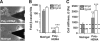
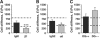
Similar articles
-
Division of labor among the alpha6beta4 integrin, beta1 integrins, and an E3 laminin receptor to signal morphogenesis and beta-casein expression in mammary epithelial cells.Mol Biol Cell. 1999 Sep;10(9):2817-28. doi: 10.1091/mbc.10.9.2817. Mol Biol Cell. 1999. PMID: 10473629 Free PMC article.
-
Laminin-rich extracellular matrix association with mammary epithelial cells suppresses Brca1 expression.Cell Death Differ. 2000 Apr;7(4):360-7. doi: 10.1038/sj.cdd.4400647. Cell Death Differ. 2000. PMID: 10773820
-
Dystroglycan loss disrupts polarity and beta-casein induction in mammary epithelial cells by perturbing laminin anchoring.J Cell Sci. 2006 Oct 1;119(Pt 19):4047-58. doi: 10.1242/jcs.03103. Epub 2006 Sep 12. J Cell Sci. 2006. PMID: 16968749 Free PMC article.
-
The influence of tissue microenvironment (stroma and extracellular matrix) on the development and function of mammary epithelium.Epithelial Cell Biol. 1993 Apr;2(2):79-89. Epithelial Cell Biol. 1993. PMID: 8353596 Review.
-
Regulation of mammary differentiation by the extracellular matrix.Environ Health Perspect. 1989 Mar;80:71-83. doi: 10.1289/ehp.898071. Environ Health Perspect. 1989. PMID: 2647486 Free PMC article. Review.
Cited by
-
Concise review: the relevance of human stem cell-derived organoid models for epithelial translational medicine.Stem Cells. 2013 Mar;31(3):417-22. doi: 10.1002/stem.1290. Stem Cells. 2013. PMID: 23203919 Free PMC article. Review.
-
Organoids as Complex In Vitro Models for Studying Radiation-Induced Cell Recruitment.Cell Mol Bioeng. 2020 Jun 15;13(4):341-357. doi: 10.1007/s12195-020-00625-0. eCollection 2020 Aug. Cell Mol Bioeng. 2020. PMID: 32952734 Free PMC article. Review.
-
Tunable synthetic extracellular matrices to investigate breast cancer response to biophysical and biochemical cues.APL Bioeng. 2019 Feb 8;3(1):016101. doi: 10.1063/1.5064596. eCollection 2019 Mar. APL Bioeng. 2019. PMID: 31069334 Free PMC article.
-
Integrated morphodynamic signalling of the mammary gland.Nat Rev Mol Cell Biol. 2011 Aug 10;12(9):581-93. doi: 10.1038/nrm3168. Nat Rev Mol Cell Biol. 2011. PMID: 21829222 Review.
-
Integrin α6 and EGFR signaling converge at mechanosensitive calpain 2.Biomaterials. 2018 Sep;178:73-82. doi: 10.1016/j.biomaterials.2018.05.056. Epub 2018 Jun 2. Biomaterials. 2018. PMID: 29909039 Free PMC article.
References
-
- Bissell MJ, Hall HG, Parry G (1982) How does the extracellular matrix direct gene expression? J Theor Biol 99: 31–68 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

