The AP-2 adaptor beta2 appendage scaffolds alternate cargo endocytosis
- PMID: 18843039
- PMCID: PMC2592659
- DOI: 10.1091/mbc.e08-07-0712
The AP-2 adaptor beta2 appendage scaffolds alternate cargo endocytosis
Abstract
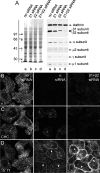
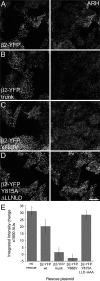
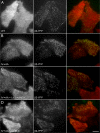
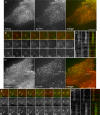
The independently folded appendages of the large alpha and beta2 subunits of the endocytic adaptor protein (AP)-2 complex coordinate proper assembly and operation of endocytic components during clathrin-mediated endocytosis. The beta2 subunit appendage contains a common binding site for beta-arrestin or the autosomal recessive hypercholesterolemia (ARH) protein. To determine the importance of this interaction surface in living cells, we used small interfering RNA-based gene silencing. The effect of extinguishing beta2 subunit expression on the internalization of transferrin is considerably weaker than an AP-2 alpha subunit knockdown. We show the mild sorting defect is due to fortuitous substitution of the beta2 chain with the closely related endogenous beta1 subunit of the AP-1 adaptor complex. Simultaneous silencing of both beta1 and beta2 subunit transcripts recapitulates the strong alpha subunit RNA interference (RNAi) phenotype and results in loss of ARH from endocytic clathrin coats. An RNAi-insensitive beta2-yellow fluorescent protein (YFP) expressed in the beta1 + beta2-silenced background restores cellular AP-2 levels, robust transferrin internalization, and ARH colocalization with cell surface clathrin. The importance of the beta appendage platform subdomain over clathrin for precise deposition of ARH at clathrin assembly zones is revealed by a beta2-YFP with a disrupted ARH binding interface, which does not restore ARH colocalization with clathrin. We also show a beta-arrestin 1 mutant, which engages coated structures in the absence of any G protein-coupled receptor stimulation, colocalizes with beta2-YFP and clathrin even in the absence of an operational clathrin binding sequence. These findings argue against ARH and beta-arrestin binding to a site upon the beta2 appendage platform that is later obstructed by polymerized clathrin. We conclude that ARH and beta-arrestin depend on a privileged beta2 appendage site for proper cargo recruitment to clathrin bud sites.
Figures


Similar articles
-
A phosphotyrosine switch for cargo sequestration at clathrin-coated buds.J Biol Chem. 2014 Jun 20;289(25):17497-514. doi: 10.1074/jbc.M114.556589. Epub 2014 May 5. J Biol Chem. 2014. PMID: 24798335 Free PMC article.
-
Functional dissection of an AP-2 beta2 appendage-binding sequence within the autosomal recessive hypercholesterolemia protein.J Biol Chem. 2005 May 13;280(19):19270-80. doi: 10.1074/jbc.M501029200. Epub 2005 Feb 22. J Biol Chem. 2005. PMID: 15728179
-
Molecular switches involving the AP-2 beta2 appendage regulate endocytic cargo selection and clathrin coat assembly.Dev Cell. 2006 Mar;10(3):329-42. doi: 10.1016/j.devcel.2006.01.016. Dev Cell. 2006. PMID: 16516836
-
Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection.J Cell Biol. 2003 Oct 27;163(2):203-8. doi: 10.1083/jcb.200309175. J Cell Biol. 2003. PMID: 14581447 Free PMC article. Review.
-
The long life of an endocytic patch that misses AP-2.Curr Genet. 2016 Nov;62(4):765-770. doi: 10.1007/s00294-016-0605-3. Epub 2016 Apr 28. Curr Genet. 2016. PMID: 27126383 Review.
Cited by
-
AP1/2β-mediated exocytosis of tapetum-specific transporters is required for pollen development in Arabidopsis thaliana.Plant Cell. 2022 Sep 27;34(10):3961-3982. doi: 10.1093/plcell/koac192. Plant Cell. 2022. PMID: 35766888 Free PMC article.
-
A phosphotyrosine switch for cargo sequestration at clathrin-coated buds.J Biol Chem. 2014 Jun 20;289(25):17497-514. doi: 10.1074/jbc.M114.556589. Epub 2014 May 5. J Biol Chem. 2014. PMID: 24798335 Free PMC article.
-
Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin.J Biol Chem. 2013 Sep 27;288(39):27849-60. doi: 10.1074/jbc.M112.445098. Epub 2013 Aug 9. J Biol Chem. 2013. PMID: 23935101 Free PMC article.
-
Clathrin regulates the association of PIPKIgamma661 with the AP-2 adaptor beta2 appendage.J Biol Chem. 2009 May 15;284(20):13924-13939. doi: 10.1074/jbc.M901017200. Epub 2009 Mar 14. J Biol Chem. 2009. PMID: 19287005 Free PMC article.
-
Retromer terminates the generation of cAMP by internalized PTH receptors.Nat Chem Biol. 2011 May;7(5):278-84. doi: 10.1038/nchembio.545. Epub 2011 Mar 27. Nat Chem Biol. 2011. PMID: 21445058 Free PMC article.
References
-
- Aguilar R. C., Ohno H., Roche K. W., Bonifacino J. S. Functional domain mapping of the clathrin-associated adaptor medium chains μ1 and μ2. J. Biol. Chem. 1997;272:27160–27166. - PubMed
-
- Bairstow S. F., Ling K., Su X., Firestone A. J., Carbonara C., Anderson R. A. Type Iγ 661 phosphatidylinositol phosphate kinase directly interacts with AP2 and regulates endocytosis. J. Biol. Chem. 2006;281:20632–20642. - PubMed
-
- Barriere H., Nemes C., Lechardeur D., Khan-Mohammad M., Fruh K., Lukacs G. L. Molecular basis of Ub-dependent internalization of membrane proteins in mammalian cells. Traffic. 2006;7:282–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

