Effects of axotomy on cultured sensory neurons of Aplysia: long-term injury-induced changes in excitability and morphology are mediated by different signaling pathways
- PMID: 18842953
- PMCID: PMC2604846
- DOI: 10.1152/jn.90539.2008
Effects of axotomy on cultured sensory neurons of Aplysia: long-term injury-induced changes in excitability and morphology are mediated by different signaling pathways
Abstract
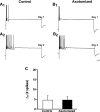
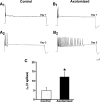
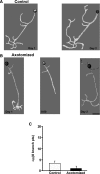
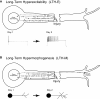
To facilitate an understanding of injury-induced changes within the nervous system, we used a single-cell, in vitro model of axonal injury. Sensory neurons were individually dissociated from the CNS of Aplysia and placed into cell culture. The major neurite of some neurons was then transected (axotomized neurons). Axotomy in hemolymph-containing culture medium produced long-term hyperexcitability (LTH-E) and enhanced neuritic sprouting (long-term hypermorphogenesis [LTH-M]). Axotomy in the absence of hemolymph induced LTH-E, but not LTH-M. Hemolymph-derived growth factors may activate tyrosine receptor kinase (Trk) receptors in sensory neurons. To examine this possibility, we treated uninjured (control) and axotomized sensory neurons with K252a, an inhibitor of Trk receptor activity. K252a depressed the excitability of both axotomized and control neurons. K252a also produced a distinct pattern of arborizing outgrowth of neurites in both axotomized and control neurons. Protein kinase C (PKC) is an intracellular signal downstream of Trk; accordingly, we tested the effects of bisindolylmaleimide I (Bis-I), a specific inhibitor of PKC, on the axotomy-induced cellular changes. Bis-I blocked LTH-E, but did not disrupt LTH-M. Finally, because Trk activates the extracellular signal regulated kinase pathway in Aplysia sensory neurons, we examined whether this pathway mediates the injury-induced changes. Sensory neurons were axotomized in the presence of U0126, an inhibitor of mitogen-activated/extracellular receptor-regulated kinase. U0126 blocked the LTH-M due to axotomy, but did not impair LTH-E. Therefore distinct cellular signaling pathways mediate the induction of LTH-E and LTH-M in the sensory neurons.
Figures
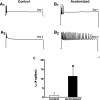
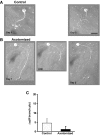
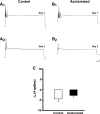
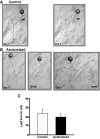


Similar articles
-
Long-term effects of axotomy on excitability and growth of isolated Aplysia sensory neurons in cell culture: potential role of cAMP.J Neurophysiol. 1998 Mar;79(3):1371-83. doi: 10.1152/jn.1998.79.3.1371. J Neurophysiol. 1998. PMID: 9497418
-
Axonal rejoining inhibits injury-induced long-term changes in Aplysia sensory neurons in vitro.J Neurosci. 2001 Dec 15;21(24):9667-77. doi: 10.1523/JNEUROSCI.21-24-09667.2001. J Neurosci. 2001. PMID: 11739576 Free PMC article.
-
Limited contributions of serotonin to long-term hyperexcitability of Aplysia sensory neurons.J Neurophysiol. 1999 Dec;82(6):3223-35. doi: 10.1152/jn.1999.82.6.3223. J Neurophysiol. 1999. PMID: 10601456
-
Pathways that elicit long-term changes in gene expression in nociceptive neurons following nerve injury: contributions to neuropathic pain.Neurol Res. 2004 Mar;26(2):195-203. doi: 10.1179/016164104225013761. Neurol Res. 2004. PMID: 15072639 Review.
-
Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture.J Neurobiol. 1994 Jun;25(6):666-93. doi: 10.1002/neu.480250608. J Neurobiol. 1994. PMID: 8071666 Review.
Cited by
-
Laminar stream of detergents for subcellular neurite damage in a microfluidic device: a simple tool for the study of neuroregeneration.J Neural Eng. 2013 Jun;10(3):036020. doi: 10.1088/1741-2560/10/3/036020. Epub 2013 May 8. J Neural Eng. 2013. PMID: 23656702 Free PMC article.
-
TRESK channel contribution to nociceptive sensory neurons excitability: modulation by nerve injury.Mol Pain. 2011 Apr 28;7:30. doi: 10.1186/1744-8069-7-30. Mol Pain. 2011. PMID: 21527011 Free PMC article.
References
-
- Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell 76: 1099–1114, 1994. - PubMed
-
- Angeles TS, Yang SX, Steffler C, Dionne CA. Kinetics of trkA tyrosine kinase activity and inhibition by K-252a. Arch Biochem Biophys 349: 267–274, 1998. - PubMed
-
- Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell 95: 211–223, 1998. - PubMed
-
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell 83: 979–992, 1995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

