Allele-specific RNA silencing of mutant ataxin-3 mediates neuroprotection in a rat model of Machado-Joseph disease
- PMID: 18841197
- PMCID: PMC2553199
- DOI: 10.1371/journal.pone.0003341
Allele-specific RNA silencing of mutant ataxin-3 mediates neuroprotection in a rat model of Machado-Joseph disease
Abstract
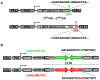
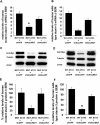
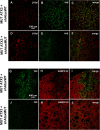
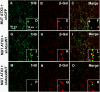
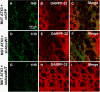
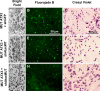
Recent studies have demonstrated that RNAi is a promising approach for treating autosomal dominant disorders. However, discrimination between wild-type and mutant transcripts is essential, to preserve wild-type expression and function. A single nucleotide polymorphism (SNP) is present in more than 70% of patients with Machado-Joseph disease (MJD). We investigated whether this SNP could be used to inactivate mutant ataxin-3 selectively. Lentiviral-mediated silencing of mutant human ataxin-3 was demonstrated in vitro and in a rat model of MJD in vivo. The allele-specific silencing of ataxin-3 significantly decreased the severity of the neuropathological abnormalities associated with MJD. These data demonstrate that RNAi has potential for use in MJD treatment and constitute the first proof-of-principle for allele-specific silencing in the central nervous system.
Conflict of interest statement
Figures


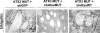
Similar articles
-
Silencing mutant ataxin-3 rescues motor deficits and neuropathology in Machado-Joseph disease transgenic mice.PLoS One. 2013;8(1):e52396. doi: 10.1371/journal.pone.0052396. Epub 2013 Jan 22. PLoS One. 2013. PMID: 23349684 Free PMC article.
-
Silencing ataxin-3 mitigates degeneration in a rat model of Machado-Joseph disease: no role for wild-type ataxin-3?Hum Mol Genet. 2010 Jun 15;19(12):2380-94. doi: 10.1093/hmg/ddq111. Epub 2010 Mar 22. Hum Mol Genet. 2010. PMID: 20308049
-
Striatal and nigral pathology in a lentiviral rat model of Machado-Joseph disease.Hum Mol Genet. 2008 Jul 15;17(14):2071-83. doi: 10.1093/hmg/ddn106. Epub 2008 Apr 1. Hum Mol Genet. 2008. PMID: 18385100
-
Machado-Joseph disease gene products carrying different carboxyl termini.Neurosci Res. 1997 Aug;28(4):373-7. doi: 10.1016/s0168-0102(97)00056-4. Neurosci Res. 1997. PMID: 9274833 Review.
-
Molecular analyses of Machado-Joseph disease.Cytogenet Genome Res. 2003;100(1-4):261-75. doi: 10.1159/000072862. Cytogenet Genome Res. 2003. PMID: 14526188 Review. No abstract available.
Cited by
-
Recent advances in RNA interference therapeutics for CNS diseases.Neurotherapeutics. 2013 Jul;10(3):473-85. doi: 10.1007/s13311-013-0183-8. Neurotherapeutics. 2013. PMID: 23589092 Free PMC article. Review.
-
Repeated Mesenchymal Stromal Cell Treatment Sustainably Alleviates Machado-Joseph Disease.Mol Ther. 2018 Sep 5;26(9):2131-2151. doi: 10.1016/j.ymthe.2018.07.007. Epub 2018 Jul 12. Mol Ther. 2018. PMID: 30087083 Free PMC article.
-
miRNA-Mediated Knockdown of ATXN3 Alleviates Molecular Disease Hallmarks in a Mouse Model for Spinocerebellar Ataxia Type 3.Nucleic Acid Ther. 2022 Jun;32(3):194-205. doi: 10.1089/nat.2021.0020. Epub 2021 Dec 7. Nucleic Acid Ther. 2022. PMID: 34878314 Free PMC article.
-
Allele-selective inhibition of ataxin-3 (ATX3) expression by antisense oligomers and duplex RNAs.Biol Chem. 2011 Apr;392(4):315-25. doi: 10.1515/BC.2011.045. Epub 2011 Feb 7. Biol Chem. 2011. PMID: 21294677 Free PMC article.
-
RNA interference mitigates motor and neuropathological deficits in a cerebellar mouse model of Machado-Joseph disease.PLoS One. 2014 Aug 21;9(8):e100086. doi: 10.1371/journal.pone.0100086. eCollection 2014. PLoS One. 2014. PMID: 25144231 Free PMC article.
References
-
- Sudarsky L, Coutinho P. Machado-Joseph disease. Clin Neurosci. 1995;3:17–22. - PubMed
-
- Gwinn-Hardy K, Singleton A, O'Suilleabhain P, Boss M, Nicholl D, et al. Spinocerebellar ataxia type 3 phenotypically resembling parkinson disease in a black family. Arch Neurol. 2001;58:296–299. - PubMed
-
- Wullner U, Reimold M, Abele M, Burk K, Minnerop M, et al. Dopamine transporter positron emission tomography in spinocerebellar ataxias type 1, 2, 3, and 6. Arch Neurol. 2005;62:1280–1285. - PubMed
-
- Alves S, Regulier E, Nascimento-Ferreira I, Hassig R, Dufour N, et al. Striatal and nigral pathology in a lentiviral rat model of Machado-Joseph disease. Hum Mol Genet 2008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

