c-FLIP knockdown induces ligand-independent DR5-, FADD-, caspase-8-, and caspase-9-dependent apoptosis in breast cancer cells
- PMID: 18840411
- PMCID: PMC2610355
- DOI: 10.1016/j.bcp.2008.09.007
c-FLIP knockdown induces ligand-independent DR5-, FADD-, caspase-8-, and caspase-9-dependent apoptosis in breast cancer cells
Abstract
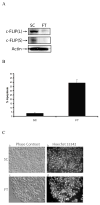
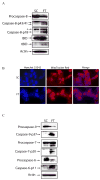
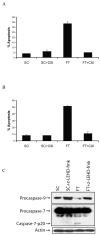
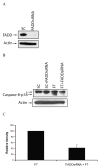
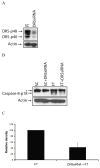
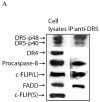
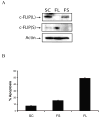
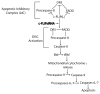
Cellular-FLICE inhibitory protein (c-FLIP) is an inhibitor of apoptosis downstream of the death receptors Fas, DR4, and DR5, and is expressed as long (c-FLIP(L)) and short (c-FLIP(S)) splice forms. We found that the knockdown of c-FLIP using small interfering RNA (siRNA) triggered ligand-independent caspase-8- and -9-dependent spontaneous apoptosis and decreased the proliferation of MCF-7 breast cancer cells. Further analysis revealed that an apoptotic inhibitory complex (AIC) comprised of DR5, FADD, caspase-8, and c-FLIP(L) exists in MCF-7 cells, and the absence of c-FLIP(L) from this complex induces DR5- and FADD-mediated caspase-8 activation in the death inducing signaling complex (DISC). c-FLIP(S) was not detected in the AIC, and using splice form-specific siRNAs we showed that c-FLIP(L) but not c-FLIP(S) is required to prevent spontaneous death signaling in MCF-7 cells. These results clearly show that c-FLIP(L) prevents ligand-independent death signaling and provides direct support for studying c-FLIP as a relevant therapeutic target for breast cancers.
Figures



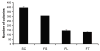
Similar articles
-
α-TEA induces apoptosis of human breast cancer cells via activation of TRAIL/DR5 death receptor pathway.Mol Carcinog. 2010 Nov;49(11):964-73. doi: 10.1002/mc.20681. Mol Carcinog. 2010. PMID: 20886583
-
c-FLIP, a master anti-apoptotic regulator.Exp Oncol. 2012 Oct;34(3):176-84. Exp Oncol. 2012. PMID: 23070002 Free PMC article. Review.
-
c-FLIP: a key regulator of colorectal cancer cell death.Cancer Res. 2007 Jun 15;67(12):5754-62. doi: 10.1158/0008-5472.CAN-06-3585. Cancer Res. 2007. PMID: 17575142
-
Fas-associated protein with death domain (FADD)-independent recruitment of c-FLIPL to death receptor 5.J Biol Chem. 2004 Dec 31;279(53):55594-601. doi: 10.1074/jbc.M401056200. Epub 2004 Oct 14. J Biol Chem. 2004. PMID: 15485835 Free PMC article.
-
Targeting c-FLIP in cancer.Cancer Lett. 2013 May 28;332(2):141-50. doi: 10.1016/j.canlet.2010.10.009. Epub 2010 Nov 10. Cancer Lett. 2013. PMID: 21071136 Review.
Cited by
-
BIN1 tumor suppressor regulates Fas/Fas ligand-mediated apoptosis through c-FLIP in cutaneous T-cell lymphoma.Leukemia. 2015 Jun;29(6):1402-13. doi: 10.1038/leu.2015.9. Epub 2015 Jan 12. Leukemia. 2015. PMID: 25578476
-
Inhibitory Effects of Total Triterpenoid Saponins Isolated from the Seeds of the Tea Plant (Camellia sinensis) on Human Ovarian Cancer Cells.Molecules. 2017 Sep 30;22(10):1649. doi: 10.3390/molecules22101649. Molecules. 2017. PMID: 28974006 Free PMC article.
-
The Crosstalk of Apoptotic and Non-Apoptotic Signaling in CD95 System.Cells. 2024 Nov 3;13(21):1814. doi: 10.3390/cells13211814. Cells. 2024. PMID: 39513921 Free PMC article. Review.
-
LUBAC Suppresses IL-21-Induced Apoptosis in CD40-Activated Murine B Cells and Promotes Germinal Center B Cell Survival and the T-Dependent Antibody Response.Front Immunol. 2021 Apr 19;12:658048. doi: 10.3389/fimmu.2021.658048. eCollection 2021. Front Immunol. 2021. PMID: 33953720 Free PMC article.
-
Role of syndecan-1 (CD138) in cell survival of human urothelial carcinoma.Cancer Sci. 2010 Jan;101(1):155-60. doi: 10.1111/j.1349-7006.2009.01379.x. Epub 2009 Oct 6. Cancer Sci. 2010. PMID: 19860843 Free PMC article.
References
-
- Cereghetti GM, Scorrano L. The many shapes of mitochondrial death. Oncogene. 2006;25:4717–24. - PubMed
-
- Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23:2950–66. - PubMed
-
- Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–71. - PubMed
-
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. - PubMed
-
- Sprick MR, Weigand MA, Rieser E, Rauch CT, Juo P, Blenis J, et al. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000;12:599–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

