Deletion of the Plasmodium falciparum merozoite surface protein 7 gene impairs parasite invasion of erythrocytes
- PMID: 18820076
- PMCID: PMC2593181
- DOI: 10.1128/EC.00274-08
Deletion of the Plasmodium falciparum merozoite surface protein 7 gene impairs parasite invasion of erythrocytes
Abstract
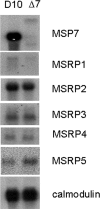
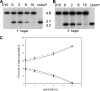
Merozoite surface proteins have been implicated in the initial attachment to the host red blood cell membrane that begins the process of invasion, an important step in the life cycle of the malaria parasite. In Plasmodium falciparum, merozoite surface proteins include several glycosylphosphatidyl inositol-anchored proteins and peripheral proteins attached to the membrane through protein-protein interactions. The most abundant of these proteins is the merozoite surface protein 1 (MSP1) complex, encoded by at least three genes: msp1, msp6, and msp7. The msp7 gene is part of a six-member multigene family in Plasmodium falciparum. We have disrupted msp7 in the Plasmodium falciparum D10 parasite, as confirmed by Southern hybridization. Immunoblot and indirect immunofluorescence analyses confirmed the MSP7 null phenotype of D10DeltaMSP7 parasites. The synthesis, distribution, and processing of MSP1 were not affected in this parasite line. The level of expression and cellular distribution of the proteins MSP1, MSP3, MSP6, MSP9, and SERA5 remained comparable to those for the parental line. Furthermore, no significant change in the expression of MSP7-related proteins, except for that of MSRP5, was detected at the transcriptional level. The lack of MSP7 was not lethal at the asexual blood stage, but it did impair invasion of erythrocytes by merozoites to a significant degree. Despite this reduction in efficiency, D10DeltaMSP7 parasites did not show any obvious preference for alternate pathways of invasion.
Figures




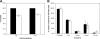
Similar articles
-
Systematic genetic analysis of the Plasmodium falciparum MSP7-like family reveals differences in protein expression, location, and importance in asexual growth of the blood-stage parasite.Eukaryot Cell. 2010 Jul;9(7):1064-74. doi: 10.1128/EC.00048-10. Epub 2010 May 14. Eukaryot Cell. 2010. PMID: 20472690 Free PMC article.
-
Merozoite surface proteins of the malaria parasite: the MSP1 complex and the MSP7 family.Int J Parasitol. 2010 Aug 15;40(10):1155-61. doi: 10.1016/j.ijpara.2010.04.008. Epub 2010 May 6. Int J Parasitol. 2010. PMID: 20451527 Review.
-
Multiple Plasmodium falciparum Merozoite Surface Protein 1 Complexes Mediate Merozoite Binding to Human Erythrocytes.J Biol Chem. 2016 Apr 1;291(14):7703-15. doi: 10.1074/jbc.M115.698282. Epub 2016 Jan 28. J Biol Chem. 2016. PMID: 26823464 Free PMC article.
-
Sequential processing of merozoite surface proteins during and after erythrocyte invasion by Plasmodium falciparum.Infect Immun. 2014 Mar;82(3):924-36. doi: 10.1128/IAI.00866-13. Epub 2013 Nov 11. Infect Immun. 2014. PMID: 24218484 Free PMC article.
-
The Cellular and Molecular Interaction Between Erythrocytes and Plasmodium falciparum Merozoites.Front Cell Infect Microbiol. 2022 Mar 31;12:816574. doi: 10.3389/fcimb.2022.816574. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35433504 Free PMC article. Review.
Cited by
-
Clinical expression and antigenic profiles of a Plasmodium vivax vaccine candidate: merozoite surface protein 7 (PvMSP-7).Malar J. 2019 Jun 13;18(1):197. doi: 10.1186/s12936-019-2826-7. Malar J. 2019. PMID: 31196098 Free PMC article.
-
Genome-wide identification and functional annotation of Plasmodium falciparum long noncoding RNAs from RNA-seq data.Parasitol Res. 2014 Apr;113(4):1269-81. doi: 10.1007/s00436-014-3765-4. Epub 2014 Feb 13. Parasitol Res. 2014. PMID: 24522451
-
Deletion of a malaria invasion gene reduces death and anemia, in model hosts.PLoS One. 2011;6(9):e25477. doi: 10.1371/journal.pone.0025477. Epub 2011 Sep 28. PLoS One. 2011. PMID: 21980474 Free PMC article.
-
Vaccination Accelerates Liver-Intrinsic Expression of Megakaryocyte-Related Genes in Response to Blood-Stage Malaria.Vaccines (Basel). 2022 Feb 14;10(2):287. doi: 10.3390/vaccines10020287. Vaccines (Basel). 2022. PMID: 35214745 Free PMC article.
-
Systematic genetic analysis of the Plasmodium falciparum MSP7-like family reveals differences in protein expression, location, and importance in asexual growth of the blood-stage parasite.Eukaryot Cell. 2010 Jul;9(7):1064-74. doi: 10.1128/EC.00048-10. Epub 2010 May 14. Eukaryot Cell. 2010. PMID: 20472690 Free PMC article.
References
-
- Abramoff, M. D., P. J. Magalhaes, and S. J. Ram. 2004. Image processing with ImageJ. Biophotonics Int. 1136-42.
-
- Bannister, L. H., G. H. Mitchell, G. A. Butcher, E. D. Dennis, and S. Cohen. 1986. Structure and development of the surface coat of erythrocytic merozoites of Plasmodium knowlesi. Cell Tissue Res. 245281-290. - PubMed
-
- Black, C. G., T. Wu, L. Wang, A. E. Topolska, and R. L. Coppel. 2005. MSP8 is a non-essential merozoite surface protein in Plasmodium falciparum. Mol. Biochem. Parasitol. 14427-35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

