The metabolism of proline as microenvironmental stress substrate
- PMID: 18806116
- PMCID: PMC2692276
- DOI: 10.1093/jn/138.10.2008S
The metabolism of proline as microenvironmental stress substrate
Abstract
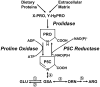
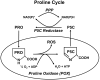
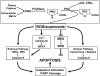
Proline, a unique proteogenic secondary amino acid, has its own metabolic system with special features. Recent findings defining the regulation of this system led us to propose that proline is a stress substrate in the microenvironment of inflammation and tumorigenesis. The criteria for proline as a stress substrate are: 1) the enzymes utilizing proline respond to stress signaling; 2) there is a large, mobilizable pool of proline; and 3) the metabolism of proline serves special stress functions. Studies show that the proline-utilizing enzyme, proline oxidase (POX)/proline dehydrogenase (PRODH), responds to genotoxic, inflammatory, and nutrient stress. Proline as substrate is stored as collagen in extracellular matrix, connective tissue, and bone and it is rapidly released from this reservoir by the sequential action of matrix metalloproteinases, peptidases, and prolidase. Special functions include the use of proline by POX/PRODH to generate superoxide radicals that initiate apoptosis by intrinsic and extrinsic pathways. Under conditions of nutrient stress, proline is an energy source. It provides carbons for the tricarboxylic acid cycle and also participates in the proline cycle. The latter, catalyzed by mitochondrial POX and cytosolic pyrroline-5-carboxylate reductase, shuttles reducing potential from the pentose phosphate pathway into mitochondria to generate ATP and oxidizing potential to activate the cytosolic pentose phosphate pathway.
Conflict of interest statement
James M. Phang, no conflicts of interest; Jui Pandhare, no conflicts of interest; Yongmin Liu, no conflicts of interest.
Figures

Similar articles
-
Proline dehydrogenase (oxidase) in cancer.Biofactors. 2012 Nov-Dec;38(6):398-406. doi: 10.1002/biof.1036. Epub 2012 Aug 8. Biofactors. 2012. PMID: 22886911 Free PMC article. Review.
-
Prolidase-proline dehydrogenase/proline oxidase-collagen biosynthesis axis as a potential interface of apoptosis/autophagy.Biofactors. 2016 Jul 8;42(4):341-8. doi: 10.1002/biof.1283. Epub 2016 Apr 4. Biofactors. 2016. PMID: 27040799 Review.
-
Understanding the role of key amino acids in regulation of proline dehydrogenase/proline oxidase (prodh/pox)-dependent apoptosis/autophagy as an approach to targeted cancer therapy.Mol Cell Biochem. 2020 Mar;466(1-2):35-44. doi: 10.1007/s11010-020-03685-y. Epub 2020 Jan 13. Mol Cell Biochem. 2020. PMID: 31933109 Free PMC article. Review.
-
Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC.Proc Natl Acad Sci U S A. 2012 Jun 5;109(23):8983-8. doi: 10.1073/pnas.1203244109. Epub 2012 May 21. Proc Natl Acad Sci U S A. 2012. PMID: 22615405 Free PMC article.
-
Collagen metabolism as a regulator of proline dehydrogenase/proline oxidase-dependent apoptosis/autophagy.Amino Acids. 2021 Dec;53(12):1917-1925. doi: 10.1007/s00726-021-02968-y. Epub 2021 Apr 5. Amino Acids. 2021. PMID: 33818628 Free PMC article. Review.
Cited by
-
Crystal structures and kinetics of monofunctional proline dehydrogenase provide insight into substrate recognition and conformational changes associated with flavin reduction and product release.Biochemistry. 2012 Dec 18;51(50):10099-108. doi: 10.1021/bi301312f. Epub 2012 Dec 5. Biochemistry. 2012. PMID: 23151026 Free PMC article.
-
In Vitro Fertilisation of Mouse Oocytes in L-Proline and L-Pipecolic Acid Improves Subsequent Development.Cells. 2021 May 29;10(6):1352. doi: 10.3390/cells10061352. Cells. 2021. PMID: 34072568 Free PMC article.
-
Distinct Metabolic Endotype Mirroring Acute Respiratory Distress Syndrome (ARDS) Subphenotype and its Heterogeneous Biology.Sci Rep. 2019 Feb 14;9(1):2108. doi: 10.1038/s41598-019-39017-4. Sci Rep. 2019. PMID: 30765824 Free PMC article.
-
The Effect of Zearalenone on the Cytokine Environment, Oxidoreductive Balance and Metabolism in Porcine Ileal Peyer's Patches.Toxins (Basel). 2020 May 27;12(6):350. doi: 10.3390/toxins12060350. Toxins (Basel). 2020. PMID: 32471145 Free PMC article.
-
Relationships between degradability of silk scaffolds and osteogenesis.Biomaterials. 2010 Aug;31(24):6162-72. doi: 10.1016/j.biomaterials.2010.04.028. Biomaterials. 2010. PMID: 20546890 Free PMC article.
References
-
- Adams E. Metabolism of proline and hydroxyproline. Int Rev Connect Tissue Res. 1970;5:1–91. - PubMed
-
- Phang JM. The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr Topics Cell Regul. 1985;25:91–132. - PubMed
-
- Phang JM, Hu CA, Valle D. Disorders of proline and hydroxyproline metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic and Molecular Basis of Inherited Disease. McGraw Hill Press; New York: 2001. pp. 1821–38.
-
- Hagedorn CH, Phang JM. Transfer of reducing equivalents into mitochondria by the Interconversions of proline and Δ1-pyrroline-5-carboxylate. Arch Biochem Biophys. 1983;225:95–101. - PubMed
-
- Adams E, Frank L. Metabolism of proline and the hydroxyprolines. Annu Rev Biochem. 1980;49:1005–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

