Reinforcement of cell junctions correlates with the absence of hair cell regeneration in mammals and its occurrence in birds
- PMID: 18803241
- PMCID: PMC2582022
- DOI: 10.1002/cne.21849
Reinforcement of cell junctions correlates with the absence of hair cell regeneration in mammals and its occurrence in birds
Erratum in
- J Comp Neurol. 2011 Dec 15;519(18):3816. Burns, Joseph [corrected to Burns, Joseph C]
Abstract
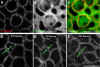
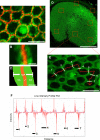
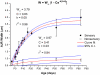
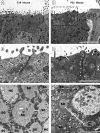
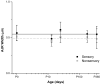
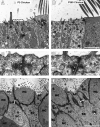
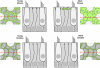
Debilitating hearing and balance deficits often arise through damage to the inner ear's hair cells. For humans and other mammals, such deficits are permanent, but nonmammalian vertebrates can quickly recover hearing and balance through their innate capacity to regenerate hair cells. The biological basis for this difference has remained unknown, but recent investigations in wounded balance epithelia have shown that proliferation follows cellular spreading at sites of injury. As mammalian ears mature during the first weeks after birth, the capacity for spreading and proliferation declines sharply. In seeking the basis for those declines, we investigated the circumferential bands of F-actin that bracket the apical junctions between supporting cells in the gravity-sensitive utricle. We found that those bands grow much thicker as mice and humans mature postnatally, whereas their counterparts in chickens remain thin from hatching through adulthood. When we cultured utricular epithelia from chickens, we found that cellular spreading and proliferation both continued at high levels, even in the epithelia from adults. In contrast, the substantial reinforcement of the circumferential F-actin bands in mammals coincides with the steep declines in cell spreading and production established in earlier experiments. We propose that the presence of thin F-actin bands at the junctions between avian supporting cells may contribute to the lifelong persistence of their capacity for shape change, cell proliferation, and hair cell replacement and that the postnatal reinforcement of the F-actin bands in maturing humans and other mammals may have an important role in limiting hair cell regeneration.
(c) 2008 Wiley-Liss, Inc.
Figures





Similar articles
-
Specializations of intercellular junctions are associated with the presence and absence of hair cell regeneration in ears from six vertebrate classes.J Comp Neurol. 2013 Apr 15;521(6):1430-48. doi: 10.1002/cne.23250. J Comp Neurol. 2013. PMID: 23124808
-
EGF and a GSK3 Inhibitor Deplete Junctional E-cadherin and Stimulate Proliferation in the Mature Mammalian Ear.J Neurosci. 2020 Mar 25;40(13):2618-2632. doi: 10.1523/JNEUROSCI.2630-19.2020. Epub 2020 Feb 20. J Neurosci. 2020. PMID: 32079647 Free PMC article.
-
Responses to cell loss become restricted as the supporting cells in mammalian vestibular organs grow thick junctional actin bands that develop high stability.J Neurosci. 2014 Jan 29;34(5):1998-2011. doi: 10.1523/JNEUROSCI.4355-13.2014. J Neurosci. 2014. PMID: 24478379 Free PMC article.
-
Hair cell regeneration in the avian auditory epithelium.Int J Dev Biol. 2007;51(6-7):633-47. doi: 10.1387/ijdb.072408js. Int J Dev Biol. 2007. PMID: 17891722 Review.
-
Comparative biology of the amniote vestibular utricle.Hear Res. 2024 Jul;448:109035. doi: 10.1016/j.heares.2024.109035. Epub 2024 May 19. Hear Res. 2024. PMID: 38763033 Review.
Cited by
-
The genetics of hair cell development and regeneration.Annu Rev Neurosci. 2013 Jul 8;36:361-81. doi: 10.1146/annurev-neuro-062012-170309. Epub 2013 May 29. Annu Rev Neurosci. 2013. PMID: 23724999 Free PMC article. Review.
-
Vestibular Deficits in Deafness: Clinical Presentation, Animal Modeling, and Treatment Solutions.Front Neurol. 2022 Apr 4;13:816534. doi: 10.3389/fneur.2022.816534. eCollection 2022. Front Neurol. 2022. PMID: 35444606 Free PMC article. Review.
-
Three-dimensional Organotypic Cultures of Vestibular and Auditory Sensory Organs.J Vis Exp. 2018 Jun 1;(136):57527. doi: 10.3791/57527. J Vis Exp. 2018. PMID: 29912206 Free PMC article.
-
In vivo proliferative regeneration of balance hair cells in newborn mice.J Neurosci. 2012 May 9;32(19):6570-7. doi: 10.1523/JNEUROSCI.6274-11.2012. J Neurosci. 2012. PMID: 22573679 Free PMC article.
-
The postnatal accumulation of junctional E-cadherin is inversely correlated with the capacity for supporting cells to convert directly into sensory hair cells in mammalian balance organs.J Neurosci. 2011 Aug 17;31(33):11855-66. doi: 10.1523/JNEUROSCI.2525-11.2011. J Neurosci. 2011. PMID: 21849546 Free PMC article.
References
-
- Bagger-Sjoback D, Anniko M. Development of intercellular junctions in the vestibular end-organ. A freeze-fracture study in the mouse. Ann Otol Rhinol Laryngol. 1984;93(1 Pt 1):89–95. - PubMed
-
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. - PubMed
-
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240(4860):1772–1774. - PubMed
-
- Cotanche DA. Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hear Res. 1987;30(2-3):181–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

