Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease
- PMID: 18801476
- PMCID: PMC2633437
- DOI: 10.1016/j.biopsych.2008.07.024
Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease
Abstract
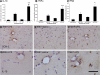
Background: Chronic neurodegeneration results in microglial activation, but the contribution of inflammation to the progress of neurodegeneration remains unclear. We have shown that microglia express low levels of proinflammatory cytokines during chronic neurodegeneration but are "primed" to produce a more proinflammatory profile after systemic challenge with bacterial endotoxin (lipopolysaccharide [LPS]).
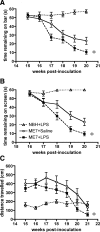
Methods: Here, we investigated whether intraperitoneal (IP) challenge with LPS, to mimic systemic infection, in the early stages of prion disease can 1) produce exaggerated acute behavioral (n = 9) and central nervous system (CNS) inflammatory (n = 4) responses in diseased animals compared with control animals, and 2) whether a single LPS challenge can accelerate disease progression (n = 34-35).
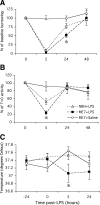
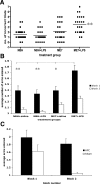
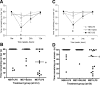
Results: Injection of LPS (100 microg/kg), at 12 weeks postinoculation (PI), resulted in heightened CNS interleukin-1 beta (IL-1beta), tumor necrosis factor-alpha (TNF-alpha), and interferon-beta (IFN-beta) transcription and microglial IL-1beta translation in prion-diseased animals relative to control animals. This inflammation caused exaggerated impairments in burrowing and locomotor activity, and induced hypothermia and cognitive changes in prion-diseased animals that were absent in LPS-treated control animals. At 15 weeks PI, LPS (500 microg/kg) acutely impaired motor coordination and muscle strength in prion-diseased but not in control animals. After recovery, these animals also showed earlier onset of disease-associated impairments on these parameters.
Conclusions: These data demonstrate that transient systemic inflammation superimposed on neurodegenerative disease acutely exacerbates cognitive and motor symptoms of disease and accelerates disease progression. These deleterious effects of systemic inflammation have implications for the treatment of chronic neurodegeneration and associated delirium.
Figures
Similar articles
-
Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNalpha/beta and IL-1beta responses in the diseased brain and exacerbates chronic neurodegeneration.Brain Behav Immun. 2010 Aug;24(6):996-1007. doi: 10.1016/j.bbi.2010.04.004. Epub 2010 Apr 23. Brain Behav Immun. 2010. PMID: 20399848 Free PMC article.
-
Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration.J Neurosci. 2005 Oct 5;25(40):9275-84. doi: 10.1523/JNEUROSCI.2614-05.2005. J Neurosci. 2005. PMID: 16207887 Free PMC article.
-
Exacerbation of CNS inflammation and neurodegeneration by systemic LPS treatment is independent of circulating IL-1β and IL-6.J Neuroinflammation. 2011 May 17;8:50. doi: 10.1186/1742-2094-8-50. J Neuroinflammation. 2011. PMID: 21586125 Free PMC article.
-
Pathophysiological and behavioral effects of systemic inflammation in aged and diseased rodents with relevance to delirium: A systematic review.Brain Behav Immun. 2017 May;62:362-381. doi: 10.1016/j.bbi.2017.01.010. Epub 2017 Jan 11. Brain Behav Immun. 2017. PMID: 28088641 Review.
-
Malaise in the water maze: untangling the effects of LPS and IL-1beta on learning and memory.Brain Behav Immun. 2008 Nov;22(8):1117-27. doi: 10.1016/j.bbi.2008.05.007. Epub 2008 Jul 21. Brain Behav Immun. 2008. PMID: 18640811 Free PMC article. Review.
Cited by
-
Neutralization of RANTES and Eotaxin Prevents the Loss of Dopaminergic Neurons in a Mouse Model of Parkinson Disease.J Biol Chem. 2016 Jul 15;291(29):15267-81. doi: 10.1074/jbc.M116.714824. Epub 2016 May 12. J Biol Chem. 2016. Retraction in: J Biol Chem. 2025 Jan;301(1):108102. doi: 10.1016/j.jbc.2024.108102. PMID: 27226559 Free PMC article. Retracted.
-
Neuropsychological Impact of West Nile Virus Infection: An Extensive Neuropsychiatric Assessment of 49 Cases in Canada.PLoS One. 2016 Jun 28;11(6):e0158364. doi: 10.1371/journal.pone.0158364. eCollection 2016. PLoS One. 2016. PMID: 27352145 Free PMC article.
-
A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-α and IL-18.J Neuroinflammation. 2012 May 29;9:101. doi: 10.1186/1742-2094-9-101. J Neuroinflammation. 2012. PMID: 22642744 Free PMC article.
-
Systemic inflammation attenuates the repair of damaged brains through reduced phagocytic activity of monocytes infiltrating the brain.Mol Brain. 2024 Jul 29;17(1):47. doi: 10.1186/s13041-024-01116-3. Mol Brain. 2024. PMID: 39075534 Free PMC article.
-
Neurological Damage by Coronaviruses: A Catastrophe in the Queue!Front Immunol. 2020 Sep 10;11:565521. doi: 10.3389/fimmu.2020.565521. eCollection 2020. Front Immunol. 2020. PMID: 33013930 Free PMC article. Review.
References
-
- Aisen P.S. The potential of anti-inflammatory drugs for the treatment of Alzheimer's disease. Lancet Neurol. 2002;1:279–284. - PubMed
-
- Firuzi O., Pratico D. Coxibs and Alzheimer's disease: Should they stay or should they go? Ann Neurol. 2006;59:219–228. - PubMed
-
- McGeer P.L., McGeer E.G. NSAIDs and Alzheimer disease: Epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28:639–647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

