Geminin is partially localized to the centrosome and plays a role in proper centrosome duplication
- PMID: 18798731
- PMCID: PMC2782310
- DOI: 10.1042/BC20080109
Geminin is partially localized to the centrosome and plays a role in proper centrosome duplication
Abstract
Background information: Centrosome duplication normally parallels with DNA replication and is responsible for correct segregation of replicated DNA into the daughter cells. Although geminin interacts with Cdt1 to prevent loading of MCMs (minichromosome maintenance proteins) on to the replication origins, inactivation of geminin nevertheless causes centrosome over-duplication in addition to the re-replication of the genome, suggesting that geminin may play a role in centrosome duplication. However, the exact mechanism by which loss of geminin affects centrosomal duplication remains unclear and the possible direct interaction of geminin with centrosomal-localized proteins is still unidentified.
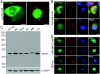
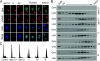
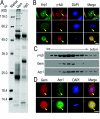
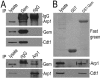
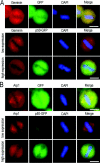
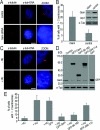
Results: We report in the present study that geminin is physically localized to the centrosome. This unexpected geminin localization is cell-cycle dependent and mediated by the actin-related protein, Arp1, one subunit of the dynein-dynactin complex. Disruption of the integrity of the dynein-dynactin complex by overexpression of dynamitin/p50, a well-characterized inhibitor of dynactin, reduces the centrosomal localization of both geminin and Arp1. Enrichment of geminin on centrosomes was enhanced when cellular ATP production was suppressed in the ATP-inhibitor assay, whereas the accumulation of geminin on the centrosome was disrupted by depolymerization of the microtubules using nocodazole. We further demonstrate that the coiled-coil motif of geminin is required for its centrosomal localization and the interaction of geminin with Arp1. Depletion of geminin by siRNA (small interfering RNA) in MDA-MB-231 cells led to centrosome over-duplication. Conversely, overexpression of geminin inhibits centrosome over-duplication induced by HU in S-phase-arrested cells, and the coiled-coil-motif-mediated centrosomal localization of geminin is required for its inhibition of centrosome over-duplication. Centrosomal localization of geminin is conserved among mammalian cells and geminin might perform as an inhibitor of centrosome duplication.
Conclusions: The results of the present study demonstrate that a fraction of geminin is localized on the centrosome, and the centrosomal localization of geminin is Arp1-mediated and dynein-dynactin-dependent. The coiled-coil motif of geminin is required for its targeting to the centrosome and inhibition of centrosome duplication. Thus the centrosomal localization of geminin might perform an important role in regulation of proper centrosome duplication.
Figures


Similar articles
-
Controlling centriole numbers: Geminin family members as master regulators of centriole amplification and multiciliogenesis.Chromosoma. 2018 Jun;127(2):151-174. doi: 10.1007/s00412-017-0652-7. Epub 2017 Dec 14. Chromosoma. 2018. PMID: 29243212 Review.
-
Nudel contributes to microtubule anchoring at the mother centriole and is involved in both dynein-dependent and -independent centrosomal protein assembly.Mol Biol Cell. 2006 Feb;17(2):680-9. doi: 10.1091/mbc.e05-04-0360. Epub 2005 Nov 16. Mol Biol Cell. 2006. PMID: 16291865 Free PMC article.
-
Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in Dictyostelium.J Cell Biol. 1999 Dec 13;147(6):1261-74. doi: 10.1083/jcb.147.6.1261. J Cell Biol. 1999. PMID: 10601339 Free PMC article.
-
Distinct cell cycle-dependent roles for dynactin and dynein at centrosomes.J Cell Biol. 2002 Oct 28;159(2):245-54. doi: 10.1083/jcb.200203089. Epub 2002 Oct 21. J Cell Biol. 2002. PMID: 12391026 Free PMC article.
-
[Dynein and dynactin as organizers of the system of cell microtubules].Ontogenez. 2006 Sep-Oct;37(5):323-39. Ontogenez. 2006. PMID: 17066975 Review. Russian.
Cited by
-
Molecular mechanism and potential target indication of TAK-931, a novel CDC7-selective inhibitor.Sci Adv. 2019 May 22;5(5):eaav3660. doi: 10.1126/sciadv.aav3660. eCollection 2019 May. Sci Adv. 2019. PMID: 31131319 Free PMC article.
-
Early S-phase cell hypersensitivity to heat stress.Cell Cycle. 2016;15(3):337-44. doi: 10.1080/15384101.2015.1127477. Epub 2015 Dec 21. Cell Cycle. 2016. PMID: 26689112 Free PMC article. Review.
-
The Replication Protein Cdc6 Suppresses Centrosome Over-Duplication in a Manner Independent of Its ATPase Activity.Mol Cells. 2017 Dec 31;40(12):925-934. doi: 10.14348/molcells.2017.0191. Epub 2017 Dec 12. Mol Cells. 2017. PMID: 29237113 Free PMC article.
-
Controlling centriole numbers: Geminin family members as master regulators of centriole amplification and multiciliogenesis.Chromosoma. 2018 Jun;127(2):151-174. doi: 10.1007/s00412-017-0652-7. Epub 2017 Dec 14. Chromosoma. 2018. PMID: 29243212 Review.
-
Geminin deletion in mouse oocytes results in impaired embryo development and reduced fertility.Mol Biol Cell. 2016 Mar 1;27(5):768-75. doi: 10.1091/mbc.E15-06-0346. Epub 2016 Jan 13. Mol Biol Cell. 2016. PMID: 26764091 Free PMC article.
References
-
- Bell S.P., Dutta A. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 2002;71:333–374. - PubMed
-
- Benjamin J.M., Torke S.J., Demeler B., McGarry T.J. Geminin has dimerization, Cdt1-binding, and destruction domains that are required for biological activity. J. Biol. Chem. 2004;279:45957–45968. - PubMed
-
- Chong J.P., Mahbubani H.M., Khoo C.Y., Blow J.J. Purification of an MCM-containing complex as a component of the DNA replication licensing system. Nature. 1995;375:418–421. - PubMed
-
- Clark S.W., Meyer D.I. Centractin is an actin homologue associated with the centrosome. Nature. 1992;359:246–250. - PubMed
-
- D'Assoro A.B., Busby R., Suino K., Delva E., Almodovar-Mercado G.J., Johnson H., Folk C., Farrugia D.J., Vasile V., Stivala F. Genotoxic stress leads to centrosome amplification in breast cancer cell lines that have an inactive G1/S cell cycle checkpoint. Oncogene. 2004;23:4068–4075. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

