Requirement of vasculogenesis and blood circulation in late stages of liver growth in zebrafish
- PMID: 18796162
- PMCID: PMC2564926
- DOI: 10.1186/1471-213X-8-84
Requirement of vasculogenesis and blood circulation in late stages of liver growth in zebrafish
Abstract
Background: Early events in vertebrate liver development have been the major focus in previous studies, however, late events of liver organogenesis remain poorly understood. Liver vasculogenesis in vertebrates occurs through the interaction of endoderm-derived liver epithelium and mesoderm-derived endothelial cells (ECs). In zebrafish, although it has been found that ECs are not required for liver budding, how and when the spatio-temporal pattern of liver growth is coordinated with ECs remains to be elucidated.
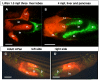
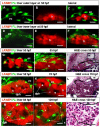
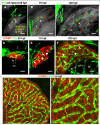
Results: To study the process of liver development and vasculogenesis in vivo, a two-color transgenic zebrafish line Tg(lfabf:dsRed; elaA:EGFP) was generated and named LiPan for liver-specific expression of DsRed RFP and exocrine pancreas-specific expression of GFP. Using the LiPan line, we first followed the dynamic development of liver from live embryos to adult and showed the formation of three distinct yet connected liver lobes during development. The LiPan line was then crossed with Tg(fli1:EGFP)y1 and vascular development in the liver was traced in vivo. Liver vasculogenesis started at 55-58 hpf when ECs first surrounded hepatocytes from the liver bud surface and then invaded the liver to form sinusoids and later the vascular network. Using a novel non-invasive and label-free fluorescence correction spectroscopy, we detected blood circulation in the liver starting at approximately 72 hpf. To analyze the roles of ECs and blood circulation in liver development, both cloche mutants (lacking ECs) and Tnnt2 morphants (no blood circulation) were employed. We found that until 70 hpf liver growth and morphogenesis depended on ECs and nascent sinusoids. After 72 hpf, a functional sinusoidal network was essential for continued liver growth. An absence of blood circulation in Tnnt2 morphants caused defects in liver vasculature and small liver.
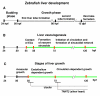
Conclusion: There are two phases of liver development in zebrafish, budding and growth. In the growth phase, there are three distinct stages: avascular growth between 50-55 hpf, where ECs are not required; endothelium-dependent growth, where ECs or sinusoids are required for liver growth between 55-72 hpf before blood circulation in liver sinusoids; and circulation-dependent growth, where the circulation is essential to maintain vascular network and to support continued liver growth after 72 hpf.
Figures



Similar articles
-
The role of vasculature and blood circulation in zebrafish swimbladder development.BMC Dev Biol. 2010 Jan 14;10:3. doi: 10.1186/1471-213X-10-3. BMC Dev Biol. 2010. PMID: 20074335 Free PMC article.
-
Visualizing the cell-cycle progression of endothelial cells in zebrafish.Dev Biol. 2014 Sep 1;393(1):10-23. doi: 10.1016/j.ydbio.2014.06.015. Epub 2014 Jun 26. Dev Biol. 2014. PMID: 24975012
-
Characterization of Endothelial Cilia Distribution During Cerebral-Vascular Development in Zebrafish ( Danio rerio).Arterioscler Thromb Vasc Biol. 2018 Dec;38(12):2806-2818. doi: 10.1161/ATVBAHA.118.311231. Arterioscler Thromb Vasc Biol. 2018. PMID: 30571172 Free PMC article.
-
What guides early embryonic blood vessel formation?Dev Dyn. 1999 May;215(1):2-11. doi: 10.1002/(SICI)1097-0177(199905)215:1<2::AID-DVDY2>3.0.CO;2-U. Dev Dyn. 1999. PMID: 10340752 Review.
-
From endoderm formation to liver and pancreas development in zebrafish.Mech Dev. 2003 Jan;120(1):5-18. doi: 10.1016/s0925-4773(02)00327-1. Mech Dev. 2003. PMID: 12490292 Review.
Cited by
-
The basic helix-loop-helix transcription factor, heart and neural crest derivatives expressed transcript 2, marks hepatic stellate cells in zebrafish: analysis of stellate cell entry into the developing liver.Hepatology. 2012 Nov;56(5):1958-70. doi: 10.1002/hep.25757. Epub 2012 Oct 14. Hepatology. 2012. PMID: 22488653 Free PMC article.
-
Anionic Lipid Nanoparticles Preferentially Deliver mRNA to the Hepatic Reticuloendothelial System.Adv Mater. 2022 Apr;34(16):e2201095. doi: 10.1002/adma.202201095. Epub 2022 Mar 10. Adv Mater. 2022. PMID: 35218106 Free PMC article.
-
BMP10-mediated ALK1 signaling is continuously required for vascular development and maintenance.Angiogenesis. 2020 May;23(2):203-220. doi: 10.1007/s10456-019-09701-0. Epub 2019 Dec 11. Angiogenesis. 2020. PMID: 31828546 Free PMC article.
-
GC-MS and LC-TOF-MS profiles, toxicity, and macrophage-dependent in vitro anti-osteoporosis activity of Prunus africana (Hook f.) Kalkman Bark.Sci Rep. 2022 Apr 29;12(1):7044. doi: 10.1038/s41598-022-10629-7. Sci Rep. 2022. PMID: 35487926 Free PMC article.
-
Zebrafish models for functional and toxicological screening of nanoscale drug delivery systems: promoting preclinical applications.Biosci Rep. 2017 Jun 8;37(3):BSR20170199. doi: 10.1042/BSR20170199. Print 2017 Jun 30. Biosci Rep. 2017. PMID: 28515222 Free PMC article. Review.
References
-
- Warga R, Nüsslein-Volhard C. Origin and development of the zebrafish endoderm. Development. 1999;126:827–838. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

