Augmentation of radiation response by panitumumab in models of upper aerodigestive tract cancer
- PMID: 18793955
- PMCID: PMC2927815
- DOI: 10.1016/j.ijrobp.2008.06.1490
Augmentation of radiation response by panitumumab in models of upper aerodigestive tract cancer
Abstract
Purpose: To examine the interaction between panitumumab, a fully human anti-epidermal growth factor receptor monoclonal antibody, and radiation in head-and-neck squamous cell carcinoma and non-small-cell lung cancer cell lines and xenografts.
Methods and materials: The head-and-neck squamous cell carcinoma lines UM-SCC1 and SCC-1483, as well as the non-small-cell lung cancer line H226, were studied. Tumor xenografts in athymic nude mice were used to assess the in vivo activity of panitumumab alone and combined with radiation. In vitro assays were performed to assess the effect of panitumumab on radiation-induced cell signaling, apoptosis, and DNA damage.
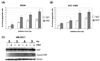
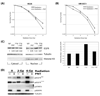
Results: Panitumumab increased the radiosensitivity as measured by the clonogenic survival assay. Radiation-induced epidermal growth factor receptor phosphorylation and downstream signaling through mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription 3 (STAT3) was inhibited by panitumumab. Panitumumab augmented radiation-induced DNA damage by 1.2-1.6-fold in each of the cell lines studied as assessed by residual gamma-H(2)AX foci after radiation. Radiation-induced apoptosis was increased 1.4-1.9-fold by panitumumab, as evidenced by Annexin V-fluorescein isothiocyanate staining and flow cytometry. In vivo, the combination therapy of panitumumab and radiation was superior to panitumumab or radiation alone in the H226 xenografts (p = 0.01) and showed a similar trend in the SCC-1483 xenografts (p = 0.08). In vivo, immunohistochemistry demonstrated the ability of panitumumab to augment the antiproliferative and antiangiogenic effects of radiation.
Conclusion: These studies have identified a favorable interaction in the combination of radiation and panitumumab in upper aerodigestive tract tumor models, both in vitro and in vivo. These data suggest that clinical investigations examining the combination of radiation and panitumumab in the treatment of epithelial tumors warrant additional pursuit.
Figures




Similar articles
-
Activity of panitumumab alone or with chemotherapy in non-small cell lung carcinoma cell lines expressing mutant epidermal growth factor receptor.Mol Cancer Ther. 2009 Jun;8(6):1536-46. doi: 10.1158/1535-7163.MCT-08-0978. Epub 2009 Jun 9. Mol Cancer Ther. 2009. PMID: 19509246
-
The effects of cetuximab alone and in combination with radiation and/or chemotherapy in lung cancer.Clin Cancer Res. 2005 Jan 15;11(2 Pt 1):795-805. Clin Cancer Res. 2005. PMID: 15701870
-
Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor.Cancer Res. 2004 Aug 1;64(15):5355-62. doi: 10.1158/0008-5472.CAN-04-0562. Cancer Res. 2004. PMID: 15289342
-
Epidermal growth factor receptors as a target for cancer treatment: the emerging role of IMC-C225 in the treatment of lung and head and neck cancers.Semin Oncol. 2002 Feb;29(1 Suppl 4):27-36. doi: 10.1053/sonc.2002.31525. Semin Oncol. 2002. PMID: 11894011 Review.
-
ZD1839, a selective epidermal growth factor receptor tyrosine kinase inhibitor, alone and in combination with radiation and chemotherapy as a new therapeutic strategy in non-small cell lung cancer.Semin Oncol. 2002 Feb;29(1 Suppl 4):37-46. doi: 10.1053/sonc.2002.31521. Semin Oncol. 2002. PMID: 11894012 Review.
Cited by
-
Autophagic action of new targeting agents in head and neck oncology.Cancer Biol Ther. 2012 Sep;13(11):978-91. doi: 10.4161/cbt.21079. Epub 2012 Jul 24. Cancer Biol Ther. 2012. PMID: 22825332 Free PMC article. Review.
-
Major Molecular Signaling Pathways in Oral Cancer Associated With Therapeutic Resistance.Front Oral Health. 2021 Jan 25;1:603160. doi: 10.3389/froh.2020.603160. eCollection 2020. Front Oral Health. 2021. PMID: 35047986 Free PMC article. Review.
-
Protein-intrinsic and signaling network-based sources of resistance to EGFR- and ErbB family-targeted therapies in head and neck cancer.Drug Resist Updat. 2011 Dec;14(6):260-79. doi: 10.1016/j.drup.2011.08.002. Epub 2011 Sep 14. Drug Resist Updat. 2011. PMID: 21920801 Free PMC article. Review.
-
Identification of differentially expressed genes associated with the enhancement of X-ray susceptibility by RITA in a hypopharyngeal squamous cell carcinoma cell line (FaDu).Radiol Oncol. 2016 Feb 22;50(2):168-74. doi: 10.1515/raon-2016-0010. eCollection 2016 Jun 1. Radiol Oncol. 2016. PMID: 27247549 Free PMC article.
-
Combining radiotherapy with targeted therapies in non-small cell lung cancer: focus on anti-EGFR, anti-ALK and anti-angiogenic agents.Transl Lung Cancer Res. 2021 Apr;10(4):2032-2047. doi: 10.21037/tlcr-20-552. Transl Lung Cancer Res. 2021. PMID: 34012812 Free PMC article. Review.
References
-
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. - PubMed
-
- Peter RU, Beetz A, Ried C, et al. Increased expression of the epidermal growth factor receptor in human epidermal keratinocytes after exposure to ionizing radiation. Radiat Res. 1993;136:65–70. - PubMed
-
- Schmidt-Ullrich RK, Valerie KC, Chan W, et al. Altered expression of epidermal growth factor receptor and estrogen receptor in MCF-7 cells after single and repeated radiation exposures. Int J Radiat Oncol Biol Phys. 1994;29:813–819. - PubMed
-
- Wollman R, Yahalom J, Maxy R, et al. Effect of epidermal growth factor on the growth and radiation sensitivity of human breast cancer cells in vitro. Int J Radiat Oncol Biol Phys. 1994;30:91–98. - PubMed
-
- Schmidt-Ullrich RK, Valerie K, Fogleman PB, et al. Radiation-induced autophosphorylation of epidermal growth factor receptor in human malignant mammary and squamous epithelial cells. Radiat Res. 1996;145:81–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA009614-12/CA/NCI NIH HHS/United States
- R01 CA113448/CA/NCI NIH HHS/United States
- T32 CA009614-16A1/CA/NCI NIH HHS/United States
- R01 CA113448-03/CA/NCI NIH HHS/United States
- T32 CA009614-15/CA/NCI NIH HHS/United States
- R01 CA113448-01A1/CA/NCI NIH HHS/United States
- T32 CA009614-17/CA/NCI NIH HHS/United States
- T32 CA009614-18/CA/NCI NIH HHS/United States
- T32 CA009614-11/CA/NCI NIH HHS/United States
- R01 CA113448-02/CA/NCI NIH HHS/United States
- T32 CA009614-13/CA/NCI NIH HHS/United States
- T32 CA009614/CA/NCI NIH HHS/United States
- R01 CA113448-05/CA/NCI NIH HHS/United States
- T32 CA009614-10/CA/NCI NIH HHS/United States
- R01 CA 113448-01/CA/NCI NIH HHS/United States
- T32 CA009614-14/CA/NCI NIH HHS/United States
- R01 CA113448-04/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

