Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling
- PMID: 18784294
- PMCID: PMC6670931
- DOI: 10.1523/JNEUROSCI.1820-08.2008
Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling
Abstract
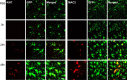
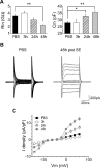
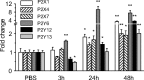
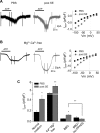
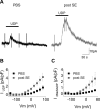
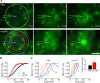
Microglia cells are the resident macrophages of the CNS, and their activation plays a critical role in inflammatory reactions associated with many brain disorders, including ischemia, Alzheimer's and Parkinson's diseases, and epilepsy. However, the changes of microglia functional properties in epilepsy have rarely been studied. Here, we used a model of status epilepticus (SE) induced by intraperitoneal kainate injections to characterize the properties of microglial cells in hippocampal slices from CX3CR1(eGFP/+) mice. SE induced within 3 h an increased expression of inflammatory mediators in the hippocampus, followed by a modification of microglia morphology, a microglia proliferation, and a significant neurodegeneration in CA1. Changes in electrophysiological intrinsic membrane properties of hippocampal microglia were detected at 24-48 h after SE with, in particular, the appearance of new voltage-activated potassium currents. Consistent with the observation of an upregulation of purinergic receptor mRNAs in the hippocampus, we also provide pharmacological evidence that microglia membrane currents mediated by the activation of P2 receptors, including P2X(7), P2Y(6), and P2Y(12), were increased 48 h after SE. As a functional consequence of this modification of purinergic signaling, motility of microglia processes toward a source of P2Y(12) receptor agonist was twice as fast in the epileptic hippocampus. This study is the first functional description of microglia activation in an in vivo model of inflammation and provides evidence for the existence of a particular microglial activation state after a status epilepticus.
Figures


Similar articles
-
Altered morphological dynamics of activated microglia after induction of status epilepticus.J Neuroinflammation. 2015 Nov 4;12:202. doi: 10.1186/s12974-015-0421-6. J Neuroinflammation. 2015. PMID: 26538404 Free PMC article.
-
Electroconvulsive Shock Enhances Responsive Motility and Purinergic Currents in Microglia in the Mouse Hippocampus.eNeuro. 2019 Apr 23;6(2):ENEURO.0056-19.2019. doi: 10.1523/ENEURO.0056-19.2019. eCollection 2019 Mar-Apr. eNeuro. 2019. PMID: 31058213 Free PMC article.
-
A postnatal peak in microglial development in the mouse hippocampus is correlated with heightened sensitivity to seizure triggers.Brain Behav. 2015 Nov 11;5(12):e00403. doi: 10.1002/brb3.403. eCollection 2015 Dec. Brain Behav. 2015. PMID: 26807334 Free PMC article.
-
Microglia and the Purinergic Signaling System.Neuroscience. 2019 May 1;405:137-147. doi: 10.1016/j.neuroscience.2018.12.021. Epub 2018 Dec 21. Neuroscience. 2019. PMID: 30582977 Review.
-
Purinergic systems in microglia.Cell Mol Life Sci. 2008 Oct;65(19):3074-80. doi: 10.1007/s00018-008-8210-3. Cell Mol Life Sci. 2008. PMID: 18563292 Free PMC article. Review.
Cited by
-
P2X7 receptor activation regulates microglial cell death during oxygen-glucose deprivation.Neuropharmacology. 2013 Oct;73:311-9. doi: 10.1016/j.neuropharm.2013.05.032. Epub 2013 Jun 12. Neuropharmacology. 2013. PMID: 23770338 Free PMC article.
-
Limited contribution of the of P2X4 receptor to LPS-induced microglial reaction in mice.Purinergic Signal. 2024 Oct;20(5):521-532. doi: 10.1007/s11302-023-09984-5. Epub 2023 Dec 30. Purinergic Signal. 2024. PMID: 38159160 Free PMC article.
-
Microglial Contact Prevents Excess Depolarization and Rescues Neurons from Excitotoxicity.eNeuro. 2016 Jun 21;3(3):ENEURO.0004-16.2016. doi: 10.1523/ENEURO.0004-16.2016. eCollection 2016 May-Jun. eNeuro. 2016. PMID: 27390772 Free PMC article.
-
Pathophysiology of astroglial purinergic signalling.Purinergic Signal. 2012 Sep;8(3):629-57. doi: 10.1007/s11302-012-9300-0. Epub 2012 May 1. Purinergic Signal. 2012. PMID: 22544529 Free PMC article. Review.
-
Histone deacetylase inhibitor SAHA attenuates post-seizure hippocampal microglia TLR4/MYD88 signaling and inhibits TLR4 gene expression via histone acetylation.BMC Neurosci. 2016 May 18;17(1):22. doi: 10.1186/s12868-016-0264-9. BMC Neurosci. 2016. PMID: 27193049 Free PMC article.
References
-
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. - PubMed
-
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. - PubMed
-
- Benkovic SA, O'Callaghan JP, Miller DB. Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic-clonic seizures. Brain Res. 2004;1024:59–76. - PubMed
-
- Benkovic SA, O'Callaghan JP, Miller DB. Regional neuropathology following kainic acid intoxication in adult and aged C57BL/6J mice. Brain Res. 2006;1070:215–231. - PubMed
-
- Bianco F, Fumagalli M, Pravettoni E, D'Ambrosi N, Volonte C, Matteoli M, Abbracchio MP, Verderio C. Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Brain Res Rev. 2005;48:144–156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
