Mitochondrial biogenesis, switching the sorting pathway of the intermembrane space receptor Mia40
- PMID: 18779329
- PMCID: PMC2662054
- DOI: 10.1074/jbc.M805356200
Mitochondrial biogenesis, switching the sorting pathway of the intermembrane space receptor Mia40
Abstract
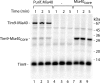
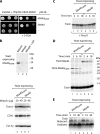
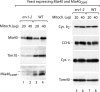
Mitochondrial precursor proteins are directed into the intermembrane space via two different routes, the presequence pathway and the redox-dependent MIA pathway. The pathways were assumed to be independent and transport different proteins. We report that the intermembrane space receptor Mia40 can switch between both pathways. In fungi, Mia40 is synthesized as large protein with an N-terminal presequence, whereas in metazoans and plants, Mia40 consists only of the conserved C-terminal domain. Human MIA40 and the C-terminal domain of yeast Mia40 (termed Mia40(core)) rescued the viability of Mia40-deficient yeast independently of the presence of a presequence. Purified Mia40(core) was imported into mitochondria via the MIA pathway. With cells expressing both full-length Mia40 and Mia40(core), we demonstrate that yeast Mia40 contains dual targeting information, directing the large precursor onto the presequence pathway and the smaller Mia40(core) onto the MIA pathway, raising interesting implications for the evolution of mitochondrial protein sorting.
Figures



Similar articles
-
Mitochondrial protein import: Mia40 facilitates Tim22 translocation into the inner membrane of mitochondria.Mol Biol Cell. 2013 Mar;24(5):543-54. doi: 10.1091/mbc.E12-09-0649. Epub 2013 Jan 2. Mol Biol Cell. 2013. PMID: 23283984 Free PMC article.
-
Disulfide bond formation: sulfhydryl oxidase ALR controls mitochondrial biogenesis of human MIA40.Traffic. 2013 Mar;14(3):309-20. doi: 10.1111/tra.12030. Epub 2012 Dec 16. Traffic. 2013. PMID: 23186364
-
Identification of the signal directing Tim9 and Tim10 into the intermembrane space of mitochondria.Mol Biol Cell. 2009 May;20(10):2530-9. doi: 10.1091/mbc.e08-11-1108. Epub 2009 Mar 18. Mol Biol Cell. 2009. PMID: 19297525 Free PMC article.
-
A disulfide relay system in mitochondria.Cell. 2005 Jul 1;121(7):965-7. doi: 10.1016/j.cell.2005.06.019. Cell. 2005. PMID: 15989945 Review.
-
AIF meets the CHCHD4/Mia40-dependent mitochondrial import pathway.Biochim Biophys Acta Mol Basis Dis. 2020 Jun 1;1866(6):165746. doi: 10.1016/j.bbadis.2020.165746. Epub 2020 Feb 24. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32105825 Review.
Cited by
-
CHCHD4 confers metabolic vulnerabilities to tumour cells through its control of the mitochondrial respiratory chain.Cancer Metab. 2019 Mar 6;7:2. doi: 10.1186/s40170-019-0194-y. eCollection 2019. Cancer Metab. 2019. PMID: 30886710 Free PMC article.
-
The C-terminal region of the oxidoreductase MIA40 stabilizes its cytosolic precursor during mitochondrial import.BMC Biol. 2020 Aug 6;18(1):96. doi: 10.1186/s12915-020-00824-1. BMC Biol. 2020. PMID: 32762682 Free PMC article.
-
Oxidative folding in the mitochondrial intermembrane space: A regulated process important for cell physiology and disease.Biochim Biophys Acta. 2016 Jun;1863(6 Pt A):1298-306. doi: 10.1016/j.bbamcr.2016.03.023. Epub 2016 Mar 28. Biochim Biophys Acta. 2016. PMID: 27033519 Free PMC article. Review.
-
Metabolic epistasis among apoptosis-inducing factor and the mitochondrial import factor CHCHD4.Cell Cycle. 2015;14(17):2743-7. doi: 10.1080/15384101.2015.1068477. Epub 2015 Jul 15. Cell Cycle. 2015. PMID: 26178476 Free PMC article.
-
Cysteine residues in mitochondrial intermembrane space proteins: more than just import.Br J Pharmacol. 2019 Feb;176(4):514-531. doi: 10.1111/bph.14480. Epub 2018 Sep 28. Br J Pharmacol. 2019. PMID: 30129023 Free PMC article. Review.
References
-
- Dolezal, P., Likic, V., Tachezy, J., and Lithgow, T. (2006) Science 313 314–318 - PubMed
-
- Oka, T., and Mihara, K. A. (2005) Mol. Cell 18 145–146 - PubMed
-
- Jensen, R. E., and Johnson, A. E. (2001) Nat. Struct. Biol. 8 1008–1010 - PubMed
-
- Neupert, W., and Herrmann, J. M. (2007) Annu. Rev. Biochem. 76 723–749 - PubMed
-
- Endo, T., Yamamoto, H., and Esaki, M. (2003) J. Cell Sci. 116 3259–3267 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

