TNF-alpha and TLR agonists increase susceptibility to HIV-1 transmission by human Langerhans cells ex vivo
- PMID: 18776939
- PMCID: PMC2528910
- DOI: 10.1172/JCI34721
TNF-alpha and TLR agonists increase susceptibility to HIV-1 transmission by human Langerhans cells ex vivo
Abstract
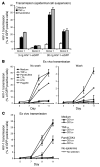
Genital coinfections increase an individual's risk of becoming infected with HIV-1 by sexual contact. Several mechanisms have been proposed to explain this, such as the presence of ulceration and bleeding caused by the coinfecting pathogen. Here we demonstrate that Langerhans cells (LCs) are involved in the increased susceptibility to HIV-1 in the presence of genital coinfections. Although LCs are a target for HIV-1 infection in genital tissues, we found that immature LCs did not efficiently mediate HIV-1 transmission in an ex vivo human skin explant model. However, the inflammatory stimuli TNF-alpha and Pam3CysSerLys4 (Pam3CSK4), the ligand for the TLR1/TLR2 heterodimer, strongly increased HIV-1 transmission by LCs through distinct mechanisms. TNF-alpha enhanced transmission by increasing HIV-1 replication in LCs, whereas Pam3CSK4 acted by increasing LC capture of HIV-1 and subsequent trans-infection of T cells. Genital infections such as Candida albicans and Neisseria gonorrhea not only triggered TLRs but also induced TNF-alpha production in vaginal and skin explants. Thus, during coinfection, LCs could be directly activated by pathogenic structures and indirectly activated by inflammatory factors, thereby increasing the risk of acquiring HIV-1. Our data demonstrate a decisive role for LCs in HIV-1 transmission during genital coinfections and suggest antiinflammatory therapies as potential strategies to prevent HIV-1 transmission.
Figures

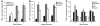
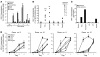
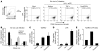
Similar articles
-
Human immature Langerhans cells restrict CXCR4-using HIV-1 transmission.Retrovirology. 2014 Jul 2;11:52. doi: 10.1186/1742-4690-11-52. Retrovirology. 2014. PMID: 24990163 Free PMC article.
-
Gram-positive bacteria enhance HIV-1 susceptibility in Langerhans cells, but not in dendritic cells, via Toll-like receptor activation.Blood. 2009 May 21;113(21):5157-66. doi: 10.1182/blood-2008-10-185728. Epub 2009 Mar 11. Blood. 2009. PMID: 19279330
-
HIV-1 exposure and immune activation enhance sexual transmission of Hepatitis C virus by primary Langerhans cells.J Int AIDS Soc. 2019 Mar;22(3):e25268. doi: 10.1002/jia2.25268. J Int AIDS Soc. 2019. PMID: 30932366 Free PMC article.
-
Human immunodeficiency virus-1 acquisition in genital mucosa: Langerhans cells as key-players.J Intern Med. 2009 Jan;265(1):18-28. doi: 10.1111/j.1365-2796.2008.02046.x. J Intern Med. 2009. PMID: 19093957 Review.
-
Langerhans cell and HIV.Nihon Rinsho Meneki Gakkai Kaishi. 2011;34(2):70-5. doi: 10.2177/jsci.34.70. Nihon Rinsho Meneki Gakkai Kaishi. 2011. PMID: 21628848 Review. English, Japanese.
Cited by
-
TLR-4 engagement of dendritic cells confers a BST-2/tetherin-mediated restriction of HIV-1 infection to CD4+ T cells across the virological synapse.Retrovirology. 2013 Jan 11;10:6. doi: 10.1186/1742-4690-10-6. Retrovirology. 2013. PMID: 23311681 Free PMC article.
-
Mucosal immunology of HIV infection.Immunol Rev. 2013 Jul;254(1):10-33. doi: 10.1111/imr.12072. Immunol Rev. 2013. PMID: 23772612 Free PMC article. Review.
-
Thymic plasmacytoid dendritic cells are susceptible to productive HIV-1 infection and efficiently transfer R5 HIV-1 to thymocytes in vitro.Retrovirology. 2011 Jun 3;8:43. doi: 10.1186/1742-4690-8-43. Retrovirology. 2011. PMID: 21639903 Free PMC article.
-
Bacterial vaginosis and the cervicovaginal immune response.Am J Reprod Immunol. 2014 Jun;71(6):555-63. doi: 10.1111/aji.12264. Am J Reprod Immunol. 2014. PMID: 24832618 Free PMC article. Review.
-
Myeloid C-Type Lectin Receptors in Viral Recognition and Antiviral Immunity.Viruses. 2017 Mar 22;9(3):59. doi: 10.3390/v9030059. Viruses. 2017. PMID: 28327518 Free PMC article. Review.
References
-
- Joint United Nations Programme on HIV/AIDS and World Health Organisation 2007. 2007 AIDS epidemic update. http://www.unaids.org .
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

