The ADAM metalloproteinases
- PMID: 18762209
- PMCID: PMC7112278
- DOI: 10.1016/j.mam.2008.08.001
The ADAM metalloproteinases
Abstract
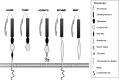
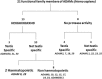
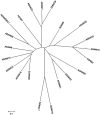
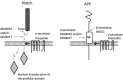
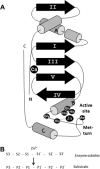
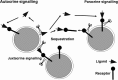
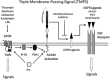
The ADAMs (a disintegrin and metalloproteinase) are a fascinating family of transmembrane and secreted proteins with important roles in regulating cell phenotype via their effects on cell adhesion, migration, proteolysis and signalling. Though all ADAMs contain metalloproteinase domains, in humans only 13 of the 21 genes in the family encode functional proteases, indicating that at least for the other eight members, protein-protein interactions are critical aspects of their biological functions. The functional ADAM metalloproteinases are involved in "ectodomain shedding" of diverse growth factors, cytokines, receptors and adhesion molecules. The archetypal activity is shown by ADAM-17 (tumour necrosis factor-alpha convertase, TACE), which is the principal protease involved in the activation of pro-TNF-alpha, but whose sheddase functions cover a broad range of cell surface molecules. In particular, ADAM-17 is required for generation of the active forms of Epidermal Growth Factor Receptor (EGFR) ligands, and its function is essential for the development of epithelial tissues. Several other ADAMs have important sheddase functions in particular tissue contexts. Another major family member, ADAM-10, is a principal player in signalling via the Notch and Eph/ephrin pathways. For a growing number of substrates, foremost among them being Notch, cleavage by ADAM sheddases is essential for their subsequent "regulated intramembrane proteolysis" (RIP), which generates cleaved intracellular domains that translocate to the nucleus and regulate gene transcription. Several ADAMs play roles in spermatogenesis and sperm function, potentially by effecting maturation of sperm and their adhesion and migration in the uterus. Other non-catalytic ADAMs function in the CNS via effects on guidance mechanisms. The ADAM family are thus fundamental to many control processes in development and homeostasis, and unsurprisingly they are also linked to pathological states when their functions are dysregulated, including cancer, cardiovascular disease, asthma, Alzheimer's disease. This review will provide an overview of current knowledge of the human ADAMs, discussing their structure, function, regulation and disease involvement.
Figures

Similar articles
-
The "a disintegrin and metalloprotease" (ADAM) family of sheddases: physiological and cellular functions.Semin Cell Dev Biol. 2009 Apr;20(2):126-37. doi: 10.1016/j.semcdb.2008.11.002. Epub 2008 Nov 13. Semin Cell Dev Biol. 2009. PMID: 19049889 Review.
-
ADAMs and protein disulfide isomerase: the key to regulated cell-surface protein ectodomain shedding?Biochem J. 2010 May 27;428(3):e3-5. doi: 10.1042/BJ20100568. Biochem J. 2010. PMID: 20504280
-
(Make) stick and cut loose--disintegrin metalloproteases in development and disease.Birth Defects Res C Embryo Today. 2006 Mar;78(1):24-46. doi: 10.1002/bdrc.20066. Birth Defects Res C Embryo Today. 2006. PMID: 16622847 Review.
-
Structural characterization of the ectodomain of a disintegrin and metalloproteinase-22 (ADAM22), a neural adhesion receptor instead of metalloproteinase: insights on ADAM function.J Biol Chem. 2009 Oct 16;284(42):29077-86. doi: 10.1074/jbc.M109.014258. Epub 2009 Aug 18. J Biol Chem. 2009. PMID: 19692335 Free PMC article.
-
The Biochemistry and Physiology of A Disintegrin and Metalloproteinases (ADAMs and ADAM-TSs) in Human Pathologies.Rev Physiol Biochem Pharmacol. 2023;184:69-120. doi: 10.1007/112_2021_67. Rev Physiol Biochem Pharmacol. 2023. PMID: 35061104 Review.
Cited by
-
The metalloprotease-disintegrin ADAM8 contributes to temozolomide chemoresistance and enhanced invasiveness of human glioblastoma cells.Neuro Oncol. 2015 Nov;17(11):1474-85. doi: 10.1093/neuonc/nov042. Epub 2015 Mar 29. Neuro Oncol. 2015. PMID: 25825051 Free PMC article.
-
A Disintegrin and Metalloproteinase10 (ADAM10) Regulates NOTCH Signaling during Early Retinal Development.PLoS One. 2016 May 25;11(5):e0156184. doi: 10.1371/journal.pone.0156184. eCollection 2016. PLoS One. 2016. PMID: 27224017 Free PMC article.
-
iRhom2: An Emerging Adaptor Regulating Immunity and Disease.Int J Mol Sci. 2020 Sep 8;21(18):6570. doi: 10.3390/ijms21186570. Int J Mol Sci. 2020. PMID: 32911849 Free PMC article. Review.
-
Adverse Cardiac Remodelling after Acute Myocardial Infarction: Old and New Biomarkers.Dis Markers. 2020 Jun 12;2020:1215802. doi: 10.1155/2020/1215802. eCollection 2020. Dis Markers. 2020. PMID: 32626540 Free PMC article. Review.
-
Increased B Cell ADAM10 in Allergic Patients and Th2 Prone Mice.PLoS One. 2015 May 1;10(5):e0124331. doi: 10.1371/journal.pone.0124331. eCollection 2015. PLoS One. 2015. PMID: 25933166 Free PMC article.
References
-
- Abram C.L., Seals D.F., Pass I., Salinsky D., Maurer L., Roth T.M., Courtneidge S.A. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J. Biol. Chem. 2003;278(19):16844–16851. - PubMed
-
- Alfandari D., Cousin H., Gaultier A., Smith K., White J.M., Darribere T., DeSimone D.W. Xenopus ADAM 13 is a metalloprotease required for cranial neural crest-cell migration. Curr. Biol. 2001;11(12):918–930. - PubMed
-
- Allinson T.M., Parkin E.T., Turner A.J., Hooper N.M. ADAMs family members as amyloid precursor protein alpha-secretases. J. Neurosci. Res. 2003;74(3):342–352. - PubMed
-
- Almeida E.A., Huovila A.P., Sutherland A.E., Stephens L.E., Calarco P.G., Shaw L.M., Mercurio A.M., Sonnenberg A., Primakoff P., Myles D.G., White J.M. Mouse egg integrin alpha 6 beta 1 functions as a sperm receptor. Cell. 1995;81(7):1095–1104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

