B cells are required for Aire-deficient mice to develop multi-organ autoinflammation: A therapeutic approach for APECED patients
- PMID: 18755889
- PMCID: PMC2529049
- DOI: 10.1073/pnas.0806874105
B cells are required for Aire-deficient mice to develop multi-organ autoinflammation: A therapeutic approach for APECED patients
Abstract
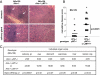
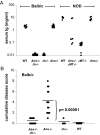
Autoimmune regulator (Aire)-deficient mice and humans have circulating autoantibodies against a multitude of organs and multiorgan autoinflammatory infiltrates. It is not known to what extent autoantibodies or their source, B lymphocytes, are required for disease onset or progression. We show in this research that B cells must be present for Aire-deficient mice to develop fulminant infiltrates. We found no evidence that autoantibodies were directly pathogenic; rather, B cells appeared to play a critical early role in T cell priming or expansion. A therapeutic reagent directed against B cells, Rituximab, induced remission of the autoimmune disease in Aire-deficient mice, raising the hope of applying it to human patients with autoimmune-polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Aire deficient mice do not develop the same profile of tissue-specific autoantibodies as APECED patients.J Autoimmun. 2006 Sep;27(2):96-104. doi: 10.1016/j.jaut.2006.06.001. Epub 2006 Jul 3. J Autoimmun. 2006. PMID: 16820279
-
Aire deficient mice develop multiple features of APECED phenotype and show altered immune response.Hum Mol Genet. 2002 Feb 15;11(4):397-409. doi: 10.1093/hmg/11.4.397. Hum Mol Genet. 2002. PMID: 11854172
-
Gastrointestinal Autoimmunity Associated With Loss of Central Tolerance to Enteric α-Defensins.Gastroenterology. 2015 Jul;149(1):139-50. doi: 10.1053/j.gastro.2015.05.009. Epub 2015 May 15. Gastroenterology. 2015. PMID: 25982289
-
Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy.J Clin Immunol. 2015 Jul;35(5):463-78. doi: 10.1007/s10875-015-0176-y. Epub 2015 Jul 5. J Clin Immunol. 2015. PMID: 26141571 Review.
-
AIRE deficiency, from preclinical models to human APECED disease.Dis Model Mech. 2021 Feb 5;14(2):dmm046359. doi: 10.1242/dmm.046359. Dis Model Mech. 2021. PMID: 33729987 Free PMC article. Review.
Cited by
-
APECED: is this a model for failure of T cell and B cell tolerance?Front Immunol. 2012 Aug 2;3:232. doi: 10.3389/fimmu.2012.00232. eCollection 2012. Front Immunol. 2012. PMID: 22876245 Free PMC article.
-
Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy.Front Pediatr. 2021 Nov 1;9:723532. doi: 10.3389/fped.2021.723532. eCollection 2021. Front Pediatr. 2021. PMID: 34790633 Free PMC article. Review.
-
B1 cells promote pancreas infiltration by autoreactive T cells.J Immunol. 2010 Sep 1;185(5):2800-7. doi: 10.4049/jimmunol.1000856. Epub 2010 Jul 30. J Immunol. 2010. PMID: 20675587 Free PMC article.
-
Aberrant type 1 immunity drives susceptibility to mucosal fungal infections.Science. 2021 Jan 15;371(6526):eaay5731. doi: 10.1126/science.aay5731. Science. 2021. PMID: 33446526 Free PMC article.
-
A Germinal Center Checkpoint of AIRE in B Cells Limits Antibody Diversification.bioRxiv [Preprint]. 2024 Jan 12:2024.01.10.574926. doi: 10.1101/2024.01.10.574926. bioRxiv. 2024. PMID: 38260362 Free PMC article. Preprint.
References
-
- Siggs OM, Makaroff LE, Liston A. The why and how of thymocyte negative selection. Curr Opin Immunol. 2006;18:175–183. - PubMed
-
- Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. - PubMed
-
- Anderson MS, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. - PubMed
-
- Anderson MS, et al. The cellular mechanism of Aire control of T cell tolerance immunity. Immunity. 2005;23:227–239. - PubMed
-
- Liston A, et al. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

