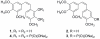Antineoplastic agents. 552. Oxidation of combretastatin A-1: trapping the o-quinone intermediate considered the metabolic product of the corresponding phosphate prodrug
- PMID: 18729517
- PMCID: PMC2756244
- DOI: 10.1021/np800179g
Antineoplastic agents. 552. Oxidation of combretastatin A-1: trapping the o-quinone intermediate considered the metabolic product of the corresponding phosphate prodrug
Abstract
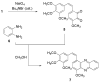
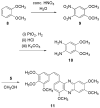
The very unstable (<10 min at rt) o-quinone 5 derived from the vicinal diphenol anticancer drug combretastatin A-1 (1) has been obtained by careful oxidation with NaIO4 and tetrabutylammonium bromide in water/dichloromethane. Immediate reaction with phenylenediamine (6) allowed o-quinone 5 to be trapped as the stable phenazine derivative 7. For further confirmation, 5 was also captured as a dimethoxyphenylenediamine-derived phenazine (11). Both phenazines 7 and 11 significantly inhibited (ED50 approximately 0.2 microg/mL) growth of the murine P388 lymphocytic leukemia cell line and provided a new SAR insight in the combretastatin series of naturally occurring anticancer drugs.
Figures
Similar articles
-
Antineoplastic agents. 578. Synthesis of stilstatins 1 and 2 and their water-soluble prodrugs.J Nat Prod. 2009 Mar 27;72(3):380-8. doi: 10.1021/np800608c. J Nat Prod. 2009. PMID: 19228038
-
Antineoplastic agents 322. synthesis of combretastatin A-4 prodrugs.Anticancer Drug Des. 1995 Jun;10(4):299-309. Anticancer Drug Des. 1995. PMID: 7786396
-
Bioreductively Activatable Prodrug Conjugates of Combretastatin A-1 and Combretastatin A-4 as Anticancer Agents Targeted toward Tumor-Associated Hypoxia.J Nat Prod. 2020 Apr 24;83(4):937-954. doi: 10.1021/acs.jnatprod.9b00773. Epub 2020 Mar 20. J Nat Prod. 2020. PMID: 32196334 Free PMC article.
-
Combretastatin A-4 analogues as antimitotic antitumor agents.Curr Med Chem. 2003 Sep;10(17):1697-722. doi: 10.2174/0929867033457151. Curr Med Chem. 2003. PMID: 12871118 Review.
-
Medicinal chemistry of combretastatin A4: present and future directions.J Med Chem. 2006 Jun 1;49(11):3033-44. doi: 10.1021/jm0512903. J Med Chem. 2006. PMID: 16722619 Review. No abstract available.
Cited by
-
Regioselective synthesis of water-soluble monophosphate derivatives of combretastatin A-1.J Nat Prod. 2011 Jul 22;74(7):1568-74. doi: 10.1021/np200104t. Epub 2011 Jun 30. J Nat Prod. 2011. PMID: 21718055 Free PMC article.
-
An efficient synthetic strategy for obtaining 4-methoxy carbon isotope labeled combretastatin A-4 phosphate and other Z-combretastatins.J Nat Prod. 2010 Mar 26;73(3):399-403. doi: 10.1021/np9004486. J Nat Prod. 2010. PMID: 20028026 Free PMC article.
-
Synthesis and cytotoxic activity of a new group of heterocyclic analogues of the combretastatins.Molecules. 2014 Jun 11;19(6):7881-900. doi: 10.3390/molecules19067881. Molecules. 2014. PMID: 24962392 Free PMC article.
-
Serial monitoring of human systemic and xenograft models of leukemia using a novel vascular disrupting agent.Leukemia. 2012 Aug;26(8):1771-8. doi: 10.1038/leu.2012.48. Epub 2012 Feb 20. Leukemia. 2012. PMID: 22343591 Free PMC article.
-
Synthesis and biological evaluation of structurally diverse α-conformationally restricted chalcones and related analogues.Medchemcomm. 2019 Jun 4;10(8):1445-1456. doi: 10.1039/c9md00127a. eCollection 2019 Aug 1. Medchemcomm. 2019. PMID: 31534659 Free PMC article.
References
-
-
Contribution 552 in the series Antineoplastic Agents: for part 551, see Pettit GR, Numata A, Iwamoto C, Usami Y, Yamada T, Ohishi H, Cragg GM. J. Nat. Prod. 2006;69:332–337.
-
-
- Pettit GR, Singh SB, Niven ML, Hamel E, Schmidt JM. J. Nat. Prod. 1987;50:119–131. - PubMed
-
- Lippert JW., III. Bioorg. Med. Chem. 2007;15:605–615. - PubMed
- Mariotti A, Perotti A, Sessa C, Rüegg C. Expert Opin. Investig. Drugs. 2007;16:451–465. - PubMed
- Vincent L, Kermani P, Young LM, Cheng J, Zhang F, Shido K, Lam G, Bompais-Vincent H, Zhu Z, Hicklin DJ, Bohlen P, Chaplin DJ, May C, Rafi S. J. Clin. Invest. 2005;115:2992–3006. - PMC - PubMed
- Deshpande HA, Gettinger SN, Sosa JA. Curr. Opin. Oncol. 2008;20:19–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous