Induction of FLIP expression by androgens protects prostate cancer cells from TRAIL-mediated apoptosis
- PMID: 18726983
- PMCID: PMC2574904
- DOI: 10.1002/pros.20844
Induction of FLIP expression by androgens protects prostate cancer cells from TRAIL-mediated apoptosis
Abstract
Background: Prostate tumors initially regress in response to androgen-ablation therapy. However, most cancers eventually relapse with an androgen-depletion-independent (ADI) phenotype that is often more aggressive than the original androgen-dependent (AD) tumor. Importantly, most relapsed tumors still rely upon androgen receptor (AR) activity for proliferation and survival. The cellular Fas/FasL-associated death domain protein-like inhibitory protein (FLIP) inhibits activation of procaspase-8 by death receptor-mediated signaling at the cell surface. In the current study, we examined the androgenic regulation of FLIP and its contribution to protecting prostate cancer cells from death receptor-mediated apoptosis.
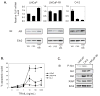
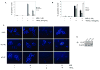
Methods: FLIP expression in tissues from intact and castrated rats as well as androgen-treated prostate cancer cell lines (LNCaP, C4-2, LNCaP-Rf, and DU-145) was monitored via Real-Time RT-PCR and immunoblot. Induction of apoptosis by TRAIL, the death receptor ligand, was determined via microscopic observation and cell counting of fragmented nuclei following fixation and staining with Hoechst 33285.
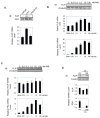
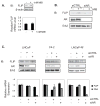
Results: FLIP mRNA and protein expression was reduced following castration in multiple rat tissues, including dorsolateral prostate and seminal vesicles. Androgenic induction of FLIP mRNA and protein was observed in isogenic AD LNCaP and ADI LNCaP-Rf cells, but not the isogenic ADI C4-2 cell line. Protection from TRAIL-induced apoptosis by androgen was completely blocked when LNCaP-Rf cells were depleted of endogenous FLIP via siRNA transfection.
Conclusions: Androgenic protection from TRAIL-induced apoptosis is predominantly via enhanced transcription of FLIP in prostate cancer cells. Loss of androgen-sensitivity in ADI prostate cancer cells highlights this pathway as a potential target for future therapy of prostate cancer.
(c) 2008 Wiley-Liss, Inc.
Figures

Similar articles
-
FOXO3a mediates the androgen-dependent regulation of FLIP and contributes to TRAIL-induced apoptosis of LNCaP cells.Oncogene. 2008 Jul 24;27(32):4422-33. doi: 10.1038/onc.2008.80. Epub 2008 Apr 7. Oncogene. 2008. PMID: 18391984
-
Interleukin-8 signaling attenuates TRAIL- and chemotherapy-induced apoptosis through transcriptional regulation of c-FLIP in prostate cancer cells.Mol Cancer Ther. 2008 Sep;7(9):2649-61. doi: 10.1158/1535-7163.MCT-08-0148. Mol Cancer Ther. 2008. PMID: 18790747
-
Low-dose 12-O-tetradecanoylphorbol-13-acetate enhances tumor necrosis factor related apoptosis-inducing ligand induced apoptosis in prostate cancer cells.Clin Cancer Res. 2007 Dec 1;13(23):7181-90. doi: 10.1158/1078-0432.CCR-07-1133. Clin Cancer Res. 2007. PMID: 18056199
-
FLIP-ping out: death receptor signaling in the prostate.Cancer Biol Ther. 2008 Aug;7(8):1171-9. doi: 10.4161/cbt.7.8.6712. Epub 2008 Aug 1. Cancer Biol Ther. 2008. PMID: 18719361 Review.
-
Targeting c-FLIP in cancer.Cancer Lett. 2013 May 28;332(2):141-50. doi: 10.1016/j.canlet.2010.10.009. Epub 2010 Nov 10. Cancer Lett. 2013. PMID: 21071136 Review.
Cited by
-
TNF is necessary for castration-induced prostate regression, whereas TRAIL and FasL are dispensable.Mol Endocrinol. 2011 Apr;25(4):611-20. doi: 10.1210/me.2010-0312. Epub 2011 Feb 3. Mol Endocrinol. 2011. PMID: 21292828 Free PMC article.
-
The natural compound atraric acid suppresses androgen-regulated neo-angiogenesis of castration-resistant prostate cancer through angiopoietin 2.Oncogene. 2022 Jun;41(23):3263-3277. doi: 10.1038/s41388-022-02333-7. Epub 2022 May 5. Oncogene. 2022. PMID: 35513564 Free PMC article.
-
Upregulation of death receptor 5 and activation of caspase 8/3 play a critical role in ergosterol peroxide induced apoptosis in DU 145 prostate cancer cells.Cancer Cell Int. 2014 Nov 30;14(1):117. doi: 10.1186/s12935-014-0117-5. eCollection 2014. Cancer Cell Int. 2014. PMID: 25506265 Free PMC article.
-
TRAIL-mediated signaling in prostate, bladder and renal cancer.Nat Rev Urol. 2011 Jun 14;8(8):417-27. doi: 10.1038/nrurol.2011.81. Nat Rev Urol. 2011. PMID: 21670755 Review.
-
Transcriptome sequencing of tumor subpopulations reveals a spectrum of therapeutic options for squamous cell lung cancer.PLoS One. 2013;8(3):e58714. doi: 10.1371/journal.pone.0058714. Epub 2013 Mar 20. PLoS One. 2013. PMID: 23527012 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. - PubMed
-
- Miyamoto H, Messing EM, Chang C. Androgen deprivation therapy for prostate cancer: Current status and future prospects. The Prostate. 2004;61(4):332–353. - PubMed
-
- Santos AF, Huang H, Tindall DJ. The androgen receptor: a potential target for therapy of prostate cancer. Steroids. 2004;69(2):79–85. - PubMed
-
- Debes JD, Tindall DJ. Mechanisms of Androgen-Refractory Prostate Cancer. New England Journal of Medicine. 2004;351(15):1488–1490. - PubMed
-
- Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. Journal of Cellular Biochemistry. 2006;99(2):333–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

