Influenza-induced expression of functional tumor necrosis factor-related apoptosis-inducing ligand on human peripheral blood mononuclear cells
- PMID: 18723061
- PMCID: PMC2597454
- DOI: 10.1016/j.humimm.2008.07.012
Influenza-induced expression of functional tumor necrosis factor-related apoptosis-inducing ligand on human peripheral blood mononuclear cells
Abstract
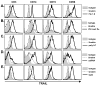
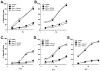
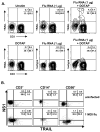
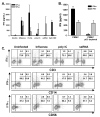
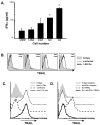
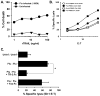
The immunologic response to influenza virus infection, like many other viruses, is characterized by robust production of proinflammatory cytokines, including type I and II interferon (IFN), which induce a number of antiviral effects and are essential for priming the innate and adaptive cellular components of the immune response. Here, we demonstrate that influenza virus infection induces the expression of functional tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on human peripheral blood mononuclear cell (PBMC) populations. Consistent with previous studies examining TRAIL upregulation, increased TRAIL expression correlated with increased type I and II IFN levels in PBMC cultures. Interestingly, dilution of these cytokines resulted in decreased expression of TRAIL. TRAIL upregulation was not dependent on active viral infection, and TRAIL was observed on NS-1-negative cells. Furthermore, influenza virus infection of lung adenocarcinoma cells (A549) resulted in increased sensitization to TRAIL-induced apoptosis compared with uninfected A549. Infected PBMC expressing TRAIL preferentially killed infected A549, but did not affect uninfected cells, and the addition of soluble TRAIL-R2:Fc blocked the lysis of infected cells, demonstrating TRAIL-dependent killing of infected cells. Collectively, these data demonstrate that TRAIL expression is induced on primary human innate and adaptive immune cells in response to cytokines produced during influenza infection and that TRAIL sensitivity is increased in influenza virus-infected cells. These data also suggest that TRAIL is a primary mechanism used by influenza-stimulated human PBMC to kill influenza-infected target cells and reinforce the importance of cytokines produced in response to TLR agonists in enhancing cellular immune effector functions.
Figures
Similar articles
-
Differential Modulation of Innate Immune Responses in Human Primary Cells by Influenza A Viruses Carrying Human or Avian Nonstructural Protein 1.J Virol. 2019 Dec 12;94(1):e00999-19. doi: 10.1128/JVI.00999-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597767 Free PMC article.
-
Role of tumor necrosis factor-related apoptosis-inducing ligand in immune response to influenza virus infection in mice.J Virol. 2005 Jun;79(12):7658-63. doi: 10.1128/JVI.79.12.7658-7663.2005. J Virol. 2005. PMID: 15919918 Free PMC article.
-
Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand.J Exp Med. 2008 Dec 22;205(13):3065-77. doi: 10.1084/jem.20080201. Epub 2008 Dec 8. J Exp Med. 2008. PMID: 19064696 Free PMC article.
-
Are blockers of gp120/CD4 interaction effective inhibitors of HIV-1 immunopathogenesis?AIDS Rev. 2006 Jan-Mar;8(1):3-8. AIDS Rev. 2006. PMID: 16736946 Review.
-
The TRAIL to viral pathogenesis: the good, the bad and the ugly.Curr Mol Med. 2009 May;9(4):495-505. doi: 10.2174/156652409788167078. Curr Mol Med. 2009. PMID: 19519406 Free PMC article. Review.
Cited by
-
Pyrogallol protects against influenza A virus-triggered lethal lung injury by activating the Nrf2-PPAR-γ-HO-1 signaling axis.MedComm (2020). 2024 Apr 12;5(4):e531. doi: 10.1002/mco2.531. eCollection 2024 Apr. MedComm (2020). 2024. PMID: 38617435 Free PMC article.
-
Divergent Roles for TRAIL in Lung Diseases.Front Med (Lausanne). 2018 Jul 27;5:212. doi: 10.3389/fmed.2018.00212. eCollection 2018. Front Med (Lausanne). 2018. PMID: 30101145 Free PMC article. Review.
-
Treating Influenza Infection, From Now and Into the Future.Front Immunol. 2018 Sep 10;9:1946. doi: 10.3389/fimmu.2018.01946. eCollection 2018. Front Immunol. 2018. PMID: 30250466 Free PMC article. Review.
-
Apoptosis and pro-inflammatory cytokine response of mast cells induced by influenza A viruses.PLoS One. 2014 Jun 12;9(6):e100109. doi: 10.1371/journal.pone.0100109. eCollection 2014. PLoS One. 2014. PMID: 24923273 Free PMC article.
-
Serum TRAIL predicts severity and prognosis in patients with community-acquired pneumonia: a prospective cohort study.Intern Emerg Med. 2022 Nov;17(8):2279-2290. doi: 10.1007/s11739-022-03086-7. Epub 2022 Oct 14. Intern Emerg Med. 2022. PMID: 36241932 Free PMC article.
References
-
- Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–200. - PubMed
-
- Doherty PC. Cytotoxic T cell effector and memory function in viral immunity. Curr Top Microbiol Immunol. 1996;206:1–14. - PubMed
-
- Lawrence CW, Braciale TJ. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J Immunol. 2004;173:1209–18. - PubMed
-
- Lawrence CW, Ream RM, Braciale TJ. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J Immunol. 2005;174:5332–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

