Nkx2-5- and Isl1-expressing cardiac progenitors contribute to proepicardium
- PMID: 18722343
- PMCID: PMC2610421
- DOI: 10.1016/j.bbrc.2008.08.044
Nkx2-5- and Isl1-expressing cardiac progenitors contribute to proepicardium
Abstract
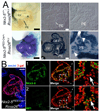
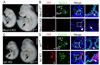
Correct delineation of the hierarchy of cardiac progenitors is a key step to understanding heart development, and will pave the way for future use of cardiac progenitors in the treatment of heart disease. Multipotent Nkx2-5 and Isl1 cardiac progenitors contribute to cardiomyocyte, smooth muscle, and endothelial lineages, which constitute the major lineages of the heart. Recently, progenitors located within the proepicardium and epicardium were reported to differentiate into cardiomyocytes, as well as smooth muscle and endothelial cells. However, the relationship of these proepicardial progenitors to the previously described Nkx2-5 and Isl1 cardiac progenitors is incompletely understood. To address this question, we performed in vivo Cre-loxP-based lineage tracing. Both Nkx2-5- and Isl1-expressing progenitors contributed to the proepicardium and expressed Wt1 and Tbx18, markers of proepicardial progenitor cells. Interestingly, Nkx2-5 knockout resulted in abnormal proepicardial development and decreased expression of Wt1, suggesting a functional role for Nkx2-5 in proepicardium formation. Taken together, these results suggest that Nkx2-5 and/or Isl1 cardiac progenitors contribute to proepicardium during heart development.
Figures

Similar articles
-
Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart.Nature. 2008 Jul 3;454(7200):109-13. doi: 10.1038/nature07060. Epub 2008 Jun 22. Nature. 2008. PMID: 18568026 Free PMC article.
-
Direct nkx2-5 transcriptional repression of isl1 controls cardiomyocyte subtype identity.Stem Cells. 2015 Apr;33(4):1113-29. doi: 10.1002/stem.1923. Stem Cells. 2015. PMID: 25524439 Free PMC article.
-
Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity.Dev Biol. 2008 Nov 1;323(1):98-104. doi: 10.1016/j.ydbio.2008.08.013. Epub 2008 Aug 22. Dev Biol. 2008. PMID: 18775691 Free PMC article.
-
Biology of Isl1+ cardiac progenitor cells in development and disease.Cell Mol Life Sci. 2007 Mar;64(6):674-82. doi: 10.1007/s00018-007-6520-5. Cell Mol Life Sci. 2007. PMID: 17380308 Free PMC article. Review.
-
The arterial and cardiac epicardium in development, disease and repair.Differentiation. 2012 Jul;84(1):41-53. doi: 10.1016/j.diff.2012.05.002. Epub 2012 May 30. Differentiation. 2012. PMID: 22652098 Review.
Cited by
-
Expandable hESC-derived cardiovascular progenitor cells generate functional cardiac lineage cells for microtissue construction.Stem Cell Res Ther. 2024 Sep 12;15(1):298. doi: 10.1186/s13287-024-03919-6. Stem Cell Res Ther. 2024. PMID: 39267174 Free PMC article.
-
Developmental origin and lineage plasticity of endogenous cardiac stem cells.Development. 2016 Apr 15;143(8):1242-58. doi: 10.1242/dev.111591. Development. 2016. PMID: 27095490 Free PMC article. Review.
-
Myocyte proliferation in the developing heart.Dev Dyn. 2011 Jun;240(6):1322-34. doi: 10.1002/dvdy.22650. Epub 2011 May 2. Dev Dyn. 2011. PMID: 21538685 Free PMC article. Review.
-
Role of c-Kit in Myocardial Regeneration and Aging.Front Endocrinol (Lausanne). 2019 Jun 19;10:371. doi: 10.3389/fendo.2019.00371. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31275242 Free PMC article. Review.
-
The Role of the Epicardium During Heart Development and Repair.Circ Res. 2020 Jan 31;126(3):377-394. doi: 10.1161/CIRCRESAHA.119.315857. Epub 2020 Jan 30. Circ Res. 2020. PMID: 31999538 Free PMC article. Review.
References
-
- Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. - PubMed
-
- Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. - PubMed
-
- Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. - PubMed
-
- Perez-Pomares JM, Carmona R, Gonzalez-Iriarte M, Atencia G, Wessels A, Munoz-Chapuli R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int J Dev Biol. 2002;46:1005–1013. - PubMed
-
- Perez-Pomares JM, Phelps A, Sedmerova M, Carmona R, Gonzalez-Iriarte M, Munoz-Chapuli R, Wessels A. Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: a model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs) Dev Biol. 2002;247:307–326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

