Tumour necrosis factor (TNF)-alpha primes murine neutrophils when triggered via formyl peptide receptor-related sequence 2, the murine orthologue of human formyl peptide receptor-like 1, through a process involving the type I TNF receptor and subcellular granule mobilization
- PMID: 18710405
- PMCID: PMC2612554
- DOI: 10.1111/j.1365-2567.2008.02873.x
Tumour necrosis factor (TNF)-alpha primes murine neutrophils when triggered via formyl peptide receptor-related sequence 2, the murine orthologue of human formyl peptide receptor-like 1, through a process involving the type I TNF receptor and subcellular granule mobilization
Abstract
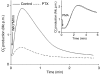
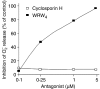
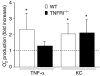
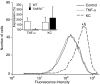
Neutrophil granulocytes play an important role in innate host defence against microbial invasions and they are also the key effector cells in mediating host tissue damage. These functions often rely on the production of reactive oxygen species (ROS) from the membrane-bound NADPH-oxidase system. The magnitude of ROS production varies depending on the state of the cells, i.e. resting or primed. Many priming agents as well as potent NADPH-oxidase activators have been identified and characterized for human neutrophils. The cytokine tumour necrosis factor (TNF)-alpha is one prominent example of a priming agent and the synthetic hexapeptide WKYMVm is an agonist that triggers an activation of the NADPH-oxidase of human neutrophils through two members of the formyl peptide family of receptors, formyl peptide receptor (FPR) and FPR-like 1 (FPRL1). This peptide also activates murine neutrophils but the precise receptor involved has not been previously characterized. We show in this study that WKYMVm activates stably transfected HL60 cells expressing murine formyl peptide receptor-related sequence 2 (Fpr-rs2) and that activation of murine neutrophils with WKYMVm is blocked by an FPRL1-specific antagonist. WKYMVm is thus an agonist for Fpr-rs2 and we suggest that this receptor is in fact the mouse orthologue of FPRL1. In addition, we show that the WKYMVm response in murine neutrophils can be primed by TNF-alpha and this priming process involves mobilization of subcellular granules. The results obtained using neutrophils derived from TNF receptor type I (TNFRI)-deficient animals suggest that TNF-alpha exerts its priming effect via the TNFRI.
Figures
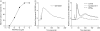
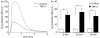
Similar articles
-
The synthetic chemoattractant Trp-Lys-Tyr-Met-Val-DMet activates neutrophils preferentially through the lipoxin A(4) receptor.Blood. 2000 Mar 1;95(5):1810-8. Blood. 2000. PMID: 10688842
-
The mechanism for activation of the neutrophil NADPH-oxidase by the peptides formyl-Met-Leu-Phe and Trp-Lys-Tyr-Met-Val-Met differs from that for interleukin-8.Immunology. 2004 Jun;112(2):201-10. doi: 10.1111/j.1365-2567.2004.01884.x. Immunology. 2004. PMID: 15147563 Free PMC article.
-
The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/lipoxin A4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2.J Biol Chem. 2001 Jun 15;276(24):21585-93. doi: 10.1074/jbc.M007769200. Epub 2001 Apr 2. J Biol Chem. 2001. PMID: 11285256
-
A new insight into the role of "old" chemotactic peptide receptors FPR and FPRL1: down-regulation of chemokine receptors CCR5 and CXCR4.Forum (Genova). 1999 Oct-Dec;9(4):299-314. Forum (Genova). 1999. PMID: 10611407 Review.
-
The potential impacts of formyl peptide receptor 1 in inflammatory diseases.Front Biosci (Elite Ed). 2016 Jun 1;8(3):436-49. doi: 10.2741/E778. Front Biosci (Elite Ed). 2016. PMID: 27100350 Review.
Cited by
-
Granule exocytosis contributes to priming and activation of the human neutrophil respiratory burst.J Immunol. 2011 Jul 1;187(1):391-400. doi: 10.4049/jimmunol.1003112. Epub 2011 Jun 3. J Immunol. 2011. PMID: 21642540 Free PMC article.
-
Data on the NADPH-oxidase activity induced by WKYMVm and galectin-3 in bone marrow derived and exudated neutrophils isolated from four different mouse strains.Data Brief. 2016 Dec 13;10:349-353. doi: 10.1016/j.dib.2016.12.010. eCollection 2017 Feb. Data Brief. 2016. PMID: 28018948 Free PMC article.
-
Human neutrophils murine neutrophils: Does it matter?Immunol Rev. 2023 Mar;314(1):442-456. doi: 10.1111/imr.13154. Epub 2022 Nov 15. Immunol Rev. 2023. PMID: 36380497 Free PMC article. Review.
-
Role of Toll-like receptor 5 in the innate immune response to acute P. aeruginosa pneumonia.Am J Physiol Lung Cell Mol Physiol. 2009 Dec;297(6):L1112-9. doi: 10.1152/ajplung.00155.2009. Epub 2009 Oct 2. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19801452 Free PMC article.
-
International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family.Pharmacol Rev. 2009 Jun;61(2):119-61. doi: 10.1124/pr.109.001578. Epub 2009 Jun 4. Pharmacol Rev. 2009. PMID: 19498085 Free PMC article. Review.
References
-
- Roos D, van Bruggen R, Meischl C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003;5:1307–15. - PubMed
-
- Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. - PubMed
-
- Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. - PubMed
-
- Betten A, Dahlgren C, Mellqvist UH, Hermodsson S, Hellstrand K. Oxygen radical-induced natural killer cell dysfunction: role of myeloperoxidase and regulation by serotonin. J Leukoc Biol. 2004;75:1111–5. - PubMed
-
- Hultqvist M, Backlund J, Bauer K, Gelderman KA, Holmdahl R. Lack of reactive oxygen species breaks T cell tolerance to collagen type II and allows development of arthritis in mice. J Immunol. 2007;179:1431–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

