C. elegans dauer formation and the molecular basis of plasticity
- PMID: 18708575
- PMCID: PMC2735354
- DOI: 10.1101/gad.1701508
C. elegans dauer formation and the molecular basis of plasticity
Abstract
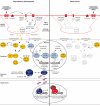
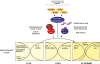
Because life is often unpredictable, dynamic, and complex, all animals have evolved remarkable abilities to cope with changes in their external environment and internal physiology. This regulatory plasticity leads to shifts in behavior and metabolism, as well as to changes in development, growth, and reproduction, which is thought to improve the chances of survival and reproductive success. In favorable environments, the nematode Caenorhabditis elegans develops rapidly to reproductive maturity, but in adverse environments, animals arrest at the dauer diapause, a long-lived stress resistant stage. A molecular and genetic analysis of dauer formation has revealed key insights into how sensory and dietary cues are coupled to conserved endocrine pathways, including insulin/IGF, TGF-beta, serotonergic, and steroid hormone signal transduction, which govern the choice between reproduction and survival. These and other pathways reveal a molecular basis for metazoan plasticity in response to extrinsic and intrinsic signals.
Figures

Similar articles
-
[Genetics and evolution of developmental plasticity in the nematode C. elegans: Environmental induction of the dauer stage].Biol Aujourdhui. 2020;214(1-2):45-53. doi: 10.1051/jbio/2020006. Epub 2020 Aug 10. Biol Aujourdhui. 2020. PMID: 32773029 Review. French.
-
Regulation of signaling genes by TGFbeta during entry into dauer diapause in C. elegans.BMC Dev Biol. 2004 Sep 20;4:11. doi: 10.1186/1471-213X-4-11. BMC Dev Biol. 2004. PMID: 15380030 Free PMC article.
-
Untangling Longevity, Dauer, and Healthspan in Caenorhabditis elegans Insulin/IGF-1-Signalling.Gerontology. 2018;64(1):96-104. doi: 10.1159/000480504. Epub 2017 Sep 22. Gerontology. 2018. PMID: 28934747 Free PMC article.
-
Endogenous RNAi Pathways Are Required in Neurons for Dauer Formation in Caenorhabditis elegans.Genetics. 2017 Apr;205(4):1503-1516. doi: 10.1534/genetics.116.195438. Epub 2017 Jan 25. Genetics. 2017. PMID: 28122825 Free PMC article.
-
Developmental plasticity and the response to nutrient stress in Caenorhabditis elegans.Dev Biol. 2021 Jul;475:265-276. doi: 10.1016/j.ydbio.2021.01.015. Epub 2021 Feb 4. Dev Biol. 2021. PMID: 33549550 Review.
Cited by
-
Co-chaperone p23 regulates C. elegans Lifespan in Response to Temperature.PLoS Genet. 2015 Apr 1;11(4):e1005023. doi: 10.1371/journal.pgen.1005023. eCollection 2015 Apr. PLoS Genet. 2015. PMID: 25830239 Free PMC article.
-
The odor of a plant metabolite affects life history traits in dietary restricted adult olive flies.Sci Rep. 2016 Jun 24;6:28540. doi: 10.1038/srep28540. Sci Rep. 2016. PMID: 27339862 Free PMC article.
-
Co-expressed Cyclin D variants cooperate to regulate proliferation of germline nuclei in a syncytium.Cell Cycle. 2015;14(13):2129-41. doi: 10.1080/15384101.2015.1041690. Epub 2015 Apr 30. Cell Cycle. 2015. PMID: 25928155 Free PMC article.
-
Acyl-CoA oxidase complexes control the chemical message produced by Caenorhabditis elegans.Proc Natl Acad Sci U S A. 2015 Mar 31;112(13):3955-60. doi: 10.1073/pnas.1423951112. Epub 2015 Mar 16. Proc Natl Acad Sci U S A. 2015. PMID: 25775534 Free PMC article.
-
Chemosensory signal transduction in Caenorhabditis elegans.Genetics. 2021 Mar 31;217(3):iyab004. doi: 10.1093/genetics/iyab004. Genetics. 2021. PMID: 33693646 Free PMC article. Review.
References
-
- Abrahante J.E., Daul A.L., Li M., Volk M.L., Tennessen J.M., Miller E.A., Rougvie A.E. The Caenorhabditis elegans hunchback-like gene lin-57/hbl-1 controls developmental time and is regulated by microRNAs. Dev. Cell. 2003;4:625–637. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
