Preventing mother-to-child transmission of HIV in resource-limited settings: the Elizabeth Glaser Pediatric AIDS Foundation experience
- PMID: 18703458
- PMCID: PMC2661479
- DOI: 10.2105/AJPH.2007.114421
Preventing mother-to-child transmission of HIV in resource-limited settings: the Elizabeth Glaser Pediatric AIDS Foundation experience
Abstract
Objectives: In September 1999, the Elizabeth Glaser Pediatric AIDS Foundation initiated a multicountry, service-based programmatic effort in the developing world to reduce perinatally acquired HIV infection. We review 6(1/2) years of one of the world's largest programs for the prevention of mother-to-child transmission (PMTCT) of HIV.
Methods: Each PMTCT facility records patient data in antenatal clinics and labor and delivery settings about counseling, testing, HIV status, and antiretroviral prophylaxis and submits the data to foundation staff.
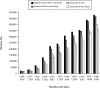
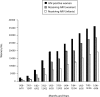
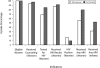
Results: More than 2.6 million women have accessed foundation-affiliated services through June 2006. Overall, 92.9% of women who received antenatal care or were eligible for PMTCT services in labor and delivery have been counseled, and 82.8% of those counseled accepted testing. Among women identified as HIV positive, 75.0% received antiretroviral prophylaxis (most a single dose of nevirapine), as did 45.6% of their infants.
Conclusions: The foundation's experience has demonstrated that opt-out testing, supplying mothers with medication at time of diagnosis, and providing the infant dose early have measurably improved program efficiency. PMTCT should be viewed as an achievable paradigm and an essential part of the continuum of care.
Figures

Similar articles
-
Integrating prevention of mother-to-child HIV transmission into routine antenatal care: the key to program expansion in Cameroon.J Acquir Immune Defic Syndr. 2005 Dec 1;40(4):486-93. doi: 10.1097/01.qai.0000163196.36199.89. J Acquir Immune Defic Syndr. 2005. PMID: 16280706
-
Voluntary counselling and testing (VCT) uptake, nevirapine use and infant feeding options at the University of Nigeria Teaching Hospital.J Obstet Gynaecol. 2008 Apr;28(3):276-9. doi: 10.1080/01443610802042639. J Obstet Gynaecol. 2008. PMID: 18569467
-
Promising outcomes of a national programme for the prevention of Mother-to-Child HIV transmission in Addis Ababa: a retrospective study.BMC Health Serv Res. 2010 Sep 9;10:267. doi: 10.1186/1472-6963-10-267. BMC Health Serv Res. 2010. PMID: 20828384 Free PMC article.
-
Lessons learned from early implementation of option B+: the Elizabeth Glaser Pediatric AIDS Foundation experience in 11 African countries.J Acquir Immune Defic Syndr. 2014 Dec 1;67 Suppl 4(Suppl 4):S188-94. doi: 10.1097/QAI.0000000000000372. J Acquir Immune Defic Syndr. 2014. PMID: 25436817 Free PMC article. Review.
-
Site-specific interventions to improve prevention of mother-to-child transmission of human immunodeficiency virus programs in less developed settings.Am J Obstet Gynecol. 2007 Sep;197(3 Suppl):S107-12. doi: 10.1016/j.ajog.2007.03.069. Am J Obstet Gynecol. 2007. PMID: 17825641 Review.
Cited by
-
Male Partner Participation in Antenatal Clinic Services is Associated With Improved HIV-Free Survival Among Infants in Nairobi, Kenya: A Prospective Cohort Study.J Acquir Immune Defic Syndr. 2016 Oct 1;73(2):169-76. doi: 10.1097/QAI.0000000000001038. J Acquir Immune Defic Syndr. 2016. PMID: 27124363 Free PMC article.
-
Inadequate coordination of maternal and infant HIV services detrimentally affects early infant diagnosis outcomes in Lilongwe, Malawi.J Acquir Immune Defic Syndr. 2011 Apr 15;56(5):e122-8. doi: 10.1097/QAI.0b013e31820a7f2f. J Acquir Immune Defic Syndr. 2011. PMID: 21224736 Free PMC article.
-
Barriers to Initiation of Pediatric HIV Treatment in Uganda: A Mixed-Method Study.AIDS Res Treat. 2012;2012:817506. doi: 10.1155/2012/817506. Epub 2012 Feb 6. AIDS Res Treat. 2012. PMID: 22400106 Free PMC article.
-
A cluster randomized controlled trial evaluating the efficacy of peer mentors to support South African women living with HIV and their infants.PLoS One. 2014 Jan 22;9(1):e84867. doi: 10.1371/journal.pone.0084867. eCollection 2014. PLoS One. 2014. PMID: 24465444 Free PMC article.
-
Optimal time on HAART for prevention of mother-to-child transmission of HIV.J Acquir Immune Defic Syndr. 2011 Oct 1;58(2):224-8. doi: 10.1097/QAI.0b013e318229147e. J Acquir Immune Defic Syndr. 2011. PMID: 21709566 Free PMC article.
References
-
- Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 1999;354:795–802 - PubMed
-
- Musoke P, Guay LA, Bagenda D, et al. A phase I/II study of the safety and pharmacokinetics of nevirapine in HIV-1-infected pregnant Ugandan women and their neonates (HIVNET 006). AIDS 1999;13:479–486 - PubMed
-
- Shaffer N, Chuachoowong R, Mock PA, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet 1999;353:773–780 - PubMed
-
- Wiktor SZ, Ekpini E, Karon JM, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Côte d'Ivoire: a randomised trial. Lancet 1999;353:781–785 - PubMed
-
- Dabis F, Msellati P, Meda N, et al. Six-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Côte d'Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. DITRAME Study Group. Diminution de la Transmission Mere-Enfant Lancet 1999;353:786–792 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

