Noroviruses distinguish between type 1 and type 2 histo-blood group antigens for binding
- PMID: 18701592
- PMCID: PMC2573190
- DOI: 10.1128/JVI.00802-08
Noroviruses distinguish between type 1 and type 2 histo-blood group antigens for binding
Abstract
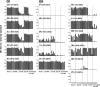
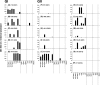
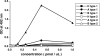
Norovirus (NoV) is a causative agent of acute gastroenteritis. NoV binds to histo-blood group antigens (HBGAs), namely, ABH antigens and Lewis (Le) antigens, in which type 1 and type 2 carbohydrate core structures constitute antigenically distinct variants. Norwalk virus, the prototype strain of norovirus, binds to the gastroduodenal junction, and this binding is correlated with the presence of H type 1 antigen but not with that of H type 2 antigen (S. Marionneau, N. Ruvoen, B. Le Moullac-Vaidye, M. Clement, A. Cailleau-Thomas, G. Ruiz-Palacois, P. Huang, X. Jiang, and J. Le Pendu, Gastroenterology 122:1967-1977, 2002). It has been unknown whether NoV distinguishes between the type 1 and type 2 chains of A and B antigens. In this study, we synthesized A type 1, A type 2, B type 1, and B type 2 pentasaccharides in vitro and examined the function of the core structures in the binding between NoV virus-like particles (VLPs) and HBGAs. The attachment of five genogroup I (GI) VLPs from 5 genotypes and 11 GII VLPs from 8 genotypes, GI/1, GI/2, GI/3, GI/4, GI/8, GII/1, GII/3, GII/4, GII/5, GII/6, GII/7, GII/12, and GII/14, to ABH and Le HBGAs was analyzed by enzyme-linked immunosorbent assay-based binding assays and Biacore analyses. GI/1, GI/2, GI/3, GI/4, GI/8, and GII/4 VLPs were more efficiently bound to A type 2 than A type 1, and GI/8 and GII/4 VLPs were more efficiently bound to B type 2 than B type 1, indicating that NoV VLPs distinguish between type 1 and type 2 carbohydrates. The dissociation of GII/4 VLPs from B type 1 was slower than that from B type 2 in the Biacore experiments; moreover, the binding to B type 1 was stronger than that to B type 2 in the ELISA experiments. These results indicated that the type 1 carbohydrates bind more tightly to NoV VLPs than the type 2 carbohydrates. This property may afford NoV tissue specificity. GII/4 is known to be a global epidemic genotype and binds to more HBGAs than other genotypes. This characteristic may be linked with the worldwide transmission of GII/4 strains. GI/2, GI/3, GI/4, GI/8, GII/4, and GII/7 VLPs bound to Le(a) expressed by nonsecretors, suggesting that NoV can infect individuals regardless of secretor phenotype. Overall, our results indicated that HBGAs are important factors in determining tissue specificity and the risk of transmission.
Figures



Similar articles
-
Norovirus and histo-blood group antigens.Jpn J Infect Dis. 2011;64(2):95-103. Jpn J Infect Dis. 2011. PMID: 21519121 Review.
-
Antibodies against Lewis antigens inhibit the binding of human norovirus GII.4 virus-like particles to saliva but not to intestinal Caco-2 cells.Virol J. 2016 May 21;13:82. doi: 10.1186/s12985-016-0538-y. Virol J. 2016. PMID: 27206610 Free PMC article.
-
Qualitative and quantitative analysis of the binding of GII.4 norovirus variants onto human blood group antigens.J Virol. 2011 May;85(9):4057-70. doi: 10.1128/JVI.02077-10. Epub 2011 Feb 23. J Virol. 2011. PMID: 21345963 Free PMC article.
-
Characterization of virus-like particles derived from a GII.3 norovirus strain distantly related with current dominating strains.Virus Genes. 2016 Oct;52(5):613-9. doi: 10.1007/s11262-016-1359-1. Epub 2016 May 27. Virus Genes. 2016. PMID: 27234312
-
[Interaction between noroviruses and human histo-blood group antigens].Uirusu. 2007 Dec;57(2):181-9. doi: 10.2222/jsv.57.181. Uirusu. 2007. PMID: 18357756 Review. Japanese.
Cited by
-
Pandemic GII.4 Sydney and Epidemic GII.17 Kawasaki308 Noroviruses Display Distinct Specificities for Histo-Blood Group Antigens Leading to Different Transmission Vector Dynamics in Pacific Oysters.Front Microbiol. 2018 Nov 27;9:2826. doi: 10.3389/fmicb.2018.02826. eCollection 2018. Front Microbiol. 2018. PMID: 30542329 Free PMC article.
-
Norovirus-host interaction: multi-selections by human histo-blood group antigens.Trends Microbiol. 2011 Aug;19(8):382-8. doi: 10.1016/j.tim.2011.05.007. Epub 2011 Jun 24. Trends Microbiol. 2011. PMID: 21705222 Free PMC article.
-
Norovirus binding to intestinal epithelial cells is independent of histo-blood group antigens.PLoS One. 2013 Jun 14;8(6):e66534. doi: 10.1371/journal.pone.0066534. Print 2013. PLoS One. 2013. PMID: 23799113 Free PMC article.
-
Epidemiology of human noroviruses and updates on vaccine development.Curr Opin Gastroenterol. 2014 Jan;30(1):25-33. doi: 10.1097/MOG.0000000000000022. Curr Opin Gastroenterol. 2014. PMID: 24232370 Free PMC article. Review.
-
Pathogenesis of noroviruses, emerging RNA viruses.Viruses. 2010 Mar;2(3):748-781. doi: 10.3390/v2030748. Epub 2010 Mar 23. Viruses. 2010. PMID: 21994656 Free PMC article.
References
-
- Bjork, S., M. E. Breimer, G. C. Hansson, K. A. Karlsson, and H. Leffler. 1987. Structures of blood group glycosphingolipids of human small intestine. A relation between the expression of fucolipids of epithelial cells and the ABO, Le and Se phenotype of the donor. J. Biol. Chem. 2626758-6765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

