The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis
- PMID: 18697942
- PMCID: PMC2575262
- DOI: 10.1073/pnas.0711697105
The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis
Abstract
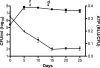
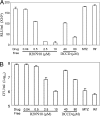
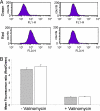
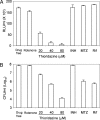
The persistence of Mycobacterium tuberculosis despite prolonged chemotherapy represents a major obstacle for the control of tuberculosis. The mechanisms used by Mtb to persist in a quiescent state are largely unknown. Chemical genetic and genetic approaches were used here to study the physiology of hypoxic nonreplicating mycobacteria. We found that the intracellular concentration of ATP is five to six times lower in hypoxic nonreplicating Mtb cells compared with aerobic replicating bacteria, making them exquisitely sensitive to any further depletion. We show that de novo ATP synthesis is essential for the viability of hypoxic nonreplicating mycobacteria, requiring the cytoplasmic membrane to be fully energized. In addition, the anaerobic electron transport chain was demonstrated to be necessary for the generation of the protonmotive force. Surprisingly, the alternate ndh-2, but not -1, was shown to be the electron donor to the electron transport chain and to be essential to replenish the [NAD(+)] pool in hypoxic nonreplicating Mtb. Finally, we describe here the high bactericidal activity of the F(0)F(1) ATP synthase inhibitor R207910 on hypoxic nonreplicating bacteria, supporting the potential of this drug candidate for shortening the time of tuberculosis therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Nutrient-starved, non-replicating Mycobacterium tuberculosis requires respiration, ATP synthase and isocitrate lyase for maintenance of ATP homeostasis and viability.Microbiology (Reading). 2010 Jan;156(Pt 1):81-87. doi: 10.1099/mic.0.033084-0. Epub 2009 Oct 1. Microbiology (Reading). 2010. PMID: 19797356
-
Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis.J Biol Chem. 2008 Sep 12;283(37):25273-25280. doi: 10.1074/jbc.M803899200. Epub 2008 Jul 14. J Biol Chem. 2008. PMID: 18625705
-
Anaerobic Mycobacterium tuberculosis Cell Death Stems from Intracellular Acidification Mitigated by the DosR Regulon.J Bacteriol. 2017 Oct 31;199(23):e00320-17. doi: 10.1128/JB.00320-17. Print 2017 Dec 1. J Bacteriol. 2017. PMID: 28874407 Free PMC article.
-
Unique structural and mechanistic properties of mycobacterial F-ATP synthases: Implications for drug design.Prog Biophys Mol Biol. 2020 May;152:64-73. doi: 10.1016/j.pbiomolbio.2019.11.006. Epub 2019 Nov 16. Prog Biophys Mol Biol. 2020. PMID: 31743686 Review.
-
Energetics of Respiration and Oxidative Phosphorylation in Mycobacteria.Microbiol Spectr. 2014 Jun;2(3):10.1128/microbiolspec.MGM2-0015-2013. doi: 10.1128/microbiolspec.MGM2-0015-2013. Microbiol Spectr. 2014. PMID: 25346874 Free PMC article. Review.
Cited by
-
Proton motive force and antibiotic tolerance in bacteria.Microb Biotechnol. 2024 Nov;17(11):e70042. doi: 10.1111/1751-7915.70042. Microb Biotechnol. 2024. PMID: 39487809 Free PMC article. Review.
-
Extensive in vivo resilience of persistent Salmonella.PLoS One. 2012;7(7):e42007. doi: 10.1371/journal.pone.0042007. Epub 2012 Jul 24. PLoS One. 2012. PMID: 22911873 Free PMC article.
-
Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis.Nat Med. 2013 Sep;19(9):1157-60. doi: 10.1038/nm.3262. Epub 2013 Aug 4. Nat Med. 2013. PMID: 23913123
-
Mycobacterium tuberculosis MT2816 encodes a key stress-response regulator.J Infect Dis. 2010 Sep 15;202(6):943-53. doi: 10.1086/654820. J Infect Dis. 2010. PMID: 20701538 Free PMC article.
-
Structure of mycobacterial respiratory complex I.Proc Natl Acad Sci U S A. 2023 Mar 28;120(13):e2214949120. doi: 10.1073/pnas.2214949120. Epub 2023 Mar 23. Proc Natl Acad Sci U S A. 2023. PMID: 36952383 Free PMC article.
References
-
- GATB. Scientific blueprint for tuberculosis drug development. Tuberculosis. 2001;81:1–52. - PubMed
-
- Mitchison DA. Shortening the treatment of tuberculosis. Nat Biotechnol. 2005;23:187–188. - PubMed
-
- Wayne LG, Sohaskey CD. Nonreplicating persistence of. Mycobacterium tuberculosis. Ann Rev Microbiol. 2001;55:139–163. - PubMed
-
- Boshoff HI, Barry CE. Tuberculosis: Metabolism and respiration in the absence of growth. Nat Rev Microbiol. 2005;3:70–80. - PubMed
-
- Dick T. Dormant tubercle bacilli: The key to more effective TB chemotherapy? J Antimicrob Chemother. 2001;47:117–118. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

