Ral-regulated interaction between Sec5 and paxillin targets Exocyst to focal complexes during cell migration
- PMID: 18697830
- PMCID: PMC4445373
- DOI: 10.1242/jcs.031641
Ral-regulated interaction between Sec5 and paxillin targets Exocyst to focal complexes during cell migration
Abstract
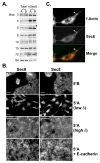
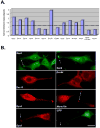
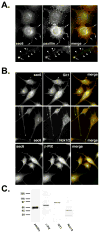
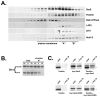
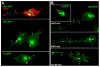
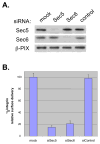
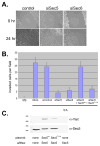
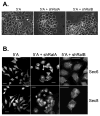
Changes in cellular behavior that cause epithelial cells to lose adhesiveness, acquire a motile invasive phenotype and metastasize to secondary sites are complex and poorly understood. Molecules that normally function to integrate adhesive spatial information with cytoskeleton dynamics and membrane trafficking probably serve important functions in cellular transformation. One such complex is the Exocyst, which is essential for targeted delivery of membrane and secretory proteins to specific plasma membrane sites to maintain epithelial cell polarity. Upon loss of cadherin-mediated adhesion in Dunning R3327-5'A prostate tumor cells, Exocyst localization shifts from lateral membranes to tips of protrusive membrane extensions. Here, it colocalizes and co-purifies with focal complex proteins that regulate membrane trafficking and cytoskeleton dynamics. These sites are the preferred destination of post-Golgi transport vesicles ferrying biosynthetic cargo, such as alpha(5)-integrin, which mediates adhesion of cells to the substratum, a process essential to cell motility. Interference with Exocyst activity impairs integrin delivery to plasma membrane and inhibits tumor cell motility and matrix invasiveness. Localization of Exocyst and, by extension, targeting of Exocyst-dependent cargo, is dependent on Ral GTPases, which control association between Sec5 and paxillin. Overexpression of Ral-uncoupled Sec5 mutants inhibited Exocyst interaction with paxillin in 5'A cells, as did RNAi-mediated reduction of either RalA or RalB. Reduction of neither GTPase significantly altered steady-state levels of assembled Exocyst in these cells, but did change the observed localization of Exocyst proteins.
Figures

Similar articles
-
Ral GTPases regulate exocyst assembly through dual subunit interactions.J Biol Chem. 2003 Dec 19;278(51):51743-8. doi: 10.1074/jbc.M308702200. Epub 2003 Oct 2. J Biol Chem. 2003. PMID: 14525976
-
The exocyst is a Ral effector complex.Nat Cell Biol. 2002 Jan;4(1):66-72. doi: 10.1038/ncb728. Nat Cell Biol. 2002. PMID: 11740492
-
RalB mobilizes the exocyst to drive cell migration.Mol Cell Biol. 2006 Jan;26(2):727-34. doi: 10.1128/MCB.26.2.727-734.2006. Mol Cell Biol. 2006. PMID: 16382162 Free PMC article.
-
The exocyst complex in exocytosis and cell migration.Protoplasma. 2012 Jul;249(3):587-97. doi: 10.1007/s00709-011-0330-1. Epub 2011 Oct 14. Protoplasma. 2012. PMID: 21997494 Review.
-
Regulation of Cell Polarity by Exocyst-Mediated Trafficking.Cold Spring Harb Perspect Biol. 2018 Mar 1;10(3):a031401. doi: 10.1101/cshperspect.a031401. Cold Spring Harb Perspect Biol. 2018. PMID: 28264817 Free PMC article. Review.
Cited by
-
Cytoplasmic cyclin D1 regulates cell invasion and metastasis through the phosphorylation of paxillin.Nat Commun. 2016 May 16;7:11581. doi: 10.1038/ncomms11581. Nat Commun. 2016. PMID: 27181366 Free PMC article.
-
The Crossroads between RAS and RHO Signaling Pathways in Cellular Transformation, Motility and Contraction.Genes (Basel). 2021 May 27;12(6):819. doi: 10.3390/genes12060819. Genes (Basel). 2021. PMID: 34071831 Free PMC article. Review.
-
Paxillin controls directional cell motility in response to physical cues.Cell Adh Migr. 2012 Nov-Dec;6(6):502-8. doi: 10.4161/cam.21672. Epub 2012 Oct 17. Cell Adh Migr. 2012. PMID: 23076140 Free PMC article.
-
The RAS-RAL axis in cancer: evidence for mutation-specific selectivity in non-small cell lung cancer.Acta Pharmacol Sin. 2015 Mar;36(3):291-7. doi: 10.1038/aps.2014.129. Epub 2015 Jan 5. Acta Pharmacol Sin. 2015. PMID: 25557115 Free PMC article. Review.
-
NAD+ biosynthesis ameliorates a zebrafish model of muscular dystrophy.PLoS Biol. 2012;10(10):e1001409. doi: 10.1371/journal.pbio.1001409. Epub 2012 Oct 23. PLoS Biol. 2012. PMID: 23109907 Free PMC article.
References
-
- Anitei M, Ifrim M, Ewart MA, Cowan AE, Carson JH, Bansal R, Pfeiffer SE. A role for Sec8 in oligodendrocyte morphological differentiation. J Cell Sci. 2006;119:807–18. - PubMed
-
- Bodemann BO, White MA. Ral GTPases and cancer: linchpin support of the tumorigenic platform. Nat Rev Cancer. 2008;8:133–40. - PubMed
-
- Bussemakers MJ, van Moorselaar RJ, Giroldi LA, Ichikawa T, Isaacs JT, Takeichi M, Debruyne FM, Schalken JA. Decreased expression of E-cadherin in the progression of rat prostatic cancer. Cancer Res. 1992;52:2916–22. - PubMed
-
- Camonis JH, White MA. Ral GTPases: corrupting the exocyst in cancer cells. Trends Cell Biol. 2005;15:327–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

