Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells
- PMID: 18695009
- PMCID: PMC2518727
- DOI: 10.1085/jgp.200810044
Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells
Abstract
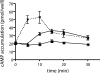
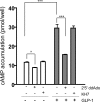
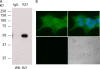
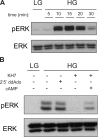
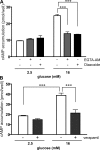
In beta cells, both glucose and hormones, such as GLP-1, stimulate production of the second messenger cAMP, but glucose and GLP-1 elicit distinct cellular responses. We now show in INS-1E insulinoma cells that glucose and GLP-1 produce cAMP with distinct kinetics via different adenylyl cyclases. GLP-1 induces a rapid cAMP signal mediated by G protein-responsive transmembrane adenylyl cyclases (tmAC). In contrast, glucose elicits a delayed cAMP rise mediated by bicarbonate, calcium, and ATP-sensitive soluble adenylyl cyclase (sAC). This glucose-induced, sAC-dependent cAMP rise is dependent upon calcium influx and is responsible for the glucose-induced activation of the mitogen-activated protein kinase (ERK1/2) pathway. These results demonstrate that sAC-generated and tmAC-generated cAMP define distinct signaling cascades.
Figures

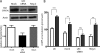

Similar articles
-
Adenylyl cyclase 8 is central to glucagon-like peptide 1 signalling and effects of chronically elevated glucose in rat and human pancreatic beta cells.Diabetologia. 2011 Feb;54(2):390-402. doi: 10.1007/s00125-010-1955-x. Epub 2010 Nov 3. Diabetologia. 2011. PMID: 21046358
-
Type VIII adenylyl cyclase in rat beta cells: coincidence signal detector/generator for glucose and GLP-1.Diabetologia. 2003 Oct;46(10):1383-93. doi: 10.1007/s00125-003-1203-8. Epub 2003 Sep 17. Diabetologia. 2003. PMID: 13680124
-
Pharmacological distinction between soluble and transmembrane adenylyl cyclases.J Pharmacol Exp Ther. 2013 Dec;347(3):589-98. doi: 10.1124/jpet.113.208496. Epub 2013 Oct 3. J Pharmacol Exp Ther. 2013. PMID: 24091307 Free PMC article.
-
The role of soluble adenylyl cyclase in sensing and regulating intracellular pH.Pflugers Arch. 2024 Apr;476(4):457-465. doi: 10.1007/s00424-024-02952-x. Epub 2024 Apr 6. Pflugers Arch. 2024. PMID: 38581526 Free PMC article. Review.
-
Central role of soluble adenylyl cyclase and cAMP in sperm physiology.Biochim Biophys Acta. 2014 Dec;1842(12 Pt B):2610-20. doi: 10.1016/j.bbadis.2014.07.013. Epub 2014 Jul 24. Biochim Biophys Acta. 2014. PMID: 25066614 Free PMC article. Review.
Cited by
-
Discovery of LRE1 as a specific and allosteric inhibitor of soluble adenylyl cyclase.Nat Chem Biol. 2016 Oct;12(10):838-44. doi: 10.1038/nchembio.2151. Epub 2016 Aug 22. Nat Chem Biol. 2016. PMID: 27547922 Free PMC article.
-
beta-Adrenergic activation of electrogenic K+ and Cl- secretion in guinea pig distal colonic epithelium proceeds via separate cAMP signaling pathways.Am J Physiol Gastrointest Liver Physiol. 2010 Jul;299(1):G81-95. doi: 10.1152/ajpgi.00035.2010. Epub 2010 Apr 22. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 20413718 Free PMC article.
-
Glucagon-Like Peptide-1 Receptor Agonist and Glucagon Increase Glucose-Stimulated Insulin Secretion in Beta Cells via Distinct Adenylyl Cyclases.Int J Med Sci. 2018 Mar 14;15(6):603-609. doi: 10.7150/ijms.24492. eCollection 2018. Int J Med Sci. 2018. PMID: 29725251 Free PMC article.
-
Regulation of epithelial Na+ transport by soluble adenylyl cyclase in kidney collecting duct cells.J Biol Chem. 2009 Feb 27;284(9):5774-83. doi: 10.1074/jbc.M805501200. Epub 2009 Jan 6. J Biol Chem. 2009. PMID: 19126549 Free PMC article.
-
Pharmacological modulation of the CO2/HCO3-/pH-, calcium-, and ATP-sensing soluble adenylyl cyclase.Pharmacol Ther. 2018 Oct;190:173-186. doi: 10.1016/j.pharmthera.2018.05.008. Epub 2018 May 26. Pharmacol Ther. 2018. PMID: 29807057 Free PMC article. Review.
References
-
- Arnette, D., T.B. Gibson, M.C. Lawrence, B. January, S. Khoo, K. McGlynn, C.A. Vanderbilt, and M.H. Cobb. 2003. Regulation of ERK1 and ERK2 by glucose and peptide hormones in pancreatic β cells. J. Biol. Chem. 278:32517–32525. - PubMed
-
- Asfari, M., D. Janjic, P. Meda, G. Li, P.A. Halban, and C.B. Wollheim. 1992. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 130:167–178. - PubMed
-
- Ashcroft, F.M., and P. Rorsman. 1989. Electrophysiology of the pancreatic β-cell. Prog. Biophys. Mol. Biol. 54:87–143. - PubMed
-
- Baillie, G.S., J.D. Scott, and M.D. Houslay. 2005. Compartmentalisation of phosphodiesterases and protein kinase A: opposites attract. FEBS Lett. 579:3264–3270. - PubMed
-
- Benes, C., M.P. Roisin, H.V. Tan, C. Creuzet, J. Miyazaki, and R. Fagard. 1998. Rapid activation and nuclear translocation of mitogen-activated protein kinases in response to physiological concentration of glucose in the MIN6 pancreatic β cell line. J. Biol. Chem. 273:15507–15513. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

