Gene profiling analysis of ALVAC infected human monocyte derived dendritic cells
- PMID: 18691624
- PMCID: PMC7115550
- DOI: 10.1016/j.vaccine.2008.07.050
Gene profiling analysis of ALVAC infected human monocyte derived dendritic cells
Abstract
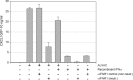
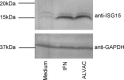
The recombinant canarypox virus ALVAC is being extensively studied as vaccine vector for the development of new vaccine strategies against chronic infectious diseases and cancer. However, the mechanisms by which ALVAC initiates the immune response have not been completely elucidated. In order to determine the type of innate immunity triggered by ALVAC, we characterized the gene expression profile of human monocyte derived dendritic cells (MDDCs) upon ALVAC infection. These cells are permissive to poxvirus infection and play a key role in the initiation of immune responses. The majority of the genes that were up-regulated by ALVAC belong to the type I interferon signaling pathway including IRF7, STAT1, RIG-1, and MDA-5. Genes involved in the NF-kappaB pathway were not up-regulated. The gene encoding for the chemokine CXCL10, a direct target of the transcription factor IRF3 was among those up-regulated and DC secretion of CXCL10 following exposure to ALVAC was confirmed by ELISA. Many downstream type I interferon activated genes with anti-viral activity (PKR, Mx, ISG15 and OAS among others) were also up-regulated in response to ALVAC. Among these, ISG15 expression in its unconjugated form by Western blot analysis was demonstrated. In view of these results we propose that ALVAC induces type I interferon anti-viral innate immunity via a cytosolic pattern-recognition-receptor (PRR) sensing double-stranded DNA, through activation of IRF3 and IRF7. These findings may aid in the design of more effective ALVAC-vectored vaccines.
Figures
Similar articles
-
A double-blind randomized phase I clinical trial targeting ALVAC-HIV vaccine to human dendritic cells.PLoS One. 2011;6(9):e24254. doi: 10.1371/journal.pone.0024254. Epub 2011 Sep 16. PLoS One. 2011. PMID: 21949699 Free PMC article. Clinical Trial.
-
Priming and Activation of Inflammasome by Canarypox Virus Vector ALVAC via the cGAS/IFI16-STING-Type I IFN Pathway and AIM2 Sensor.J Immunol. 2017 Nov 1;199(9):3293-3305. doi: 10.4049/jimmunol.1700698. Epub 2017 Sep 25. J Immunol. 2017. PMID: 28947539 Free PMC article.
-
Role of cell signaling in poxvirus-mediated foreign gene expression in mammalian cells.Vaccine. 2009 May 14;27(22):2994-3006. doi: 10.1016/j.vaccine.2009.02.103. Epub 2009 Mar 10. Vaccine. 2009. PMID: 19428911 Free PMC article.
-
CD40L expressed from the canarypox vector, ALVAC, can boost immunogenicity of HIV-1 canarypox vaccine in mice and enhance the in vitro expansion of viral specific CD8+ T cell memory responses from HIV-1-infected and HIV-1-uninfected individuals.Vaccine. 2008 Jul 29;26(32):4062-72. doi: 10.1016/j.vaccine.2008.05.018. Epub 2008 Jun 2. Vaccine. 2008. PMID: 18562053 Free PMC article.
-
Beyond ISGlylation: Functions of Free Intracellular and Extracellular ISG15.J Interferon Cytokine Res. 2017 Jun;37(6):246-253. doi: 10.1089/jir.2016.0103. Epub 2017 May 3. J Interferon Cytokine Res. 2017. PMID: 28467275 Review.
Cited by
-
Reappraising the Value of HIV-1 Vaccine Correlates of Protection Analyses.J Virol. 2022 Apr 27;96(8):e0003422. doi: 10.1128/jvi.00034-22. Epub 2022 Apr 6. J Virol. 2022. PMID: 35384694 Free PMC article. Review.
-
Different Expression of Interferon-Stimulated Genes in Response to HIV-1 Infection in Dendritic Cells Based on Their Maturation State.J Virol. 2017 Mar 29;91(8):e01379-16. doi: 10.1128/JVI.01379-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148784 Free PMC article.
-
A System Based-Approach to Examine Cytokine Response in Poxvirus-Infected Macrophages.Viruses. 2018 Dec 5;10(12):692. doi: 10.3390/v10120692. Viruses. 2018. PMID: 30563103 Free PMC article.
-
Nonhuman primate models for HIV/AIDS vaccine development.Curr Protoc Immunol. 2013 Oct 1;102:12.14.1-12.14.30. doi: 10.1002/0471142735.im1214s102. Curr Protoc Immunol. 2013. PMID: 24510515 Free PMC article. Review.
-
NOD-like receptors and RIG-I-like receptors in human eosinophils: activation by NOD1 and NOD2 agonists.Immunology. 2011 Nov;134(3):314-25. doi: 10.1111/j.1365-2567.2011.03492.x. Immunology. 2011. PMID: 21978001 Free PMC article.
References
-
- Marovich M.A. ALVAC-HIV vaccines: clinical trial experience focusing on progress in vaccine development. Expert Rev Vaccines. 2004;3(4 Suppl.):S99–S104. - PubMed
-
- de Bruyn G., Rossini A.J., Chiu Y.L., Holman D., Elizaga M.L., Frey S.E. Safety profile of recombinant canarypox HIV vaccines. Vaccine. 2004;22(5–6):704–713. - PubMed
-
- Van der Burg S.H., Menon A.G., Redeker A., Bonnet M.C., Drijfhout J.W., Tollenaar R.A. Induction of p53-specific immune responses in colorectal cancer patients receiving a recombinant ALVAC-p53 candidate vaccine. Clin Cancer Res. 2002;8(5):1019–1027. - PubMed
-
- van Baren N., Bonnet M.C., Dreno B., Khammari A., Dorval T., Piperno-Neumann S. Tumoral and immunologic response after vaccination of melanoma patients with an ALVAC virus encoding MAGE antigens recognized by T cells. J Clin Oncol. 2005;23(35):9008–9021. - PubMed
-
- Tartaglia J., Bonnet M.C., Berinstein N., Barber B., Klein M., Moingeon P. Therapeutic vaccines against melanoma and colorectal cancer. Vaccine. 2001;19(17–19):2571–2575. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

