Effect of tiotropium on health-related quality of life as a primary efficacy endpoint in COPD
- PMID: 18686739
- PMCID: PMC2629970
- DOI: 10.2147/copd.s2463
Effect of tiotropium on health-related quality of life as a primary efficacy endpoint in COPD
Abstract
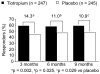
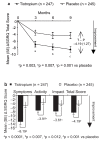
Clinical manifestations of chronic obstructive pulmonary disease (COPD), including airflow limitation, dyspnea, and activity limitation, ultimately lead to impaired health-related quality of life (HRQoL). This 9-month, randomized, double-blind, multicenter study compared the effect of once-daily tiotropium 18 microg and placebo on HRQoL, spirometric parameters, and exacerbations in 554 patients with moderate-to-severe COPD. HRQoL was assessed using the St. George's Respiratory Questionnaire (SGRQ) and the new 8-item Visual Simplified Respiratory Questionnaire (VSRQ), which is currently being validated. The primary efficacy endpoint was the proportion of patients achieving a reduction of at least 4 units in the SGRQ total score at study end (Month 9). Mean +/- SD baseline SGRQ total score was 47.4 +/- 18.1. Significantly more tiotropium-treated patients achieved a reduction of at least 4 units in the SGRQ score vs placebo at study end (59.1% vs 48.2%, respectively; p = 0.029). Tiotropium significantly improved spirometric parameters (forced expiratory volume in 1 second [FEV1]: 0.11 +/- 0.02 L vs 0.01 +/- 0.02 L; between-group difference: 0.10 +/- 0.03 L, p = 0.0001) and reduced exacerbations vs placebo. Maintenance treatment with tiotropium provided significant and clinically relevant improvements in HRQoL, as measured by the SGRQ.
Figures
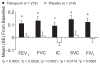
Similar articles
-
Tiotropium and exercise training in COPD patients: effects on dyspnea and exercise tolerance.Int J Chron Obstruct Pulmon Dis. 2008;3(4):771-80. doi: 10.2147/copd.s3935. Int J Chron Obstruct Pulmon Dis. 2008. PMID: 19281092 Free PMC article. Clinical Trial.
-
SOSPES: SPIRIVA® observational study measuring SGRQ score in routine medical practice in Central and Eastern Europe.Int J Chron Obstruct Pulmon Dis. 2013;8:483-92. doi: 10.2147/COPD.S45640. Epub 2013 Oct 9. Int J Chron Obstruct Pulmon Dis. 2013. PMID: 24159258 Free PMC article.
-
Outcomes in COPD patients receiving tiotropium or salmeterol plus treatment with inhaled corticosteroids.Int J Chron Obstruct Pulmon Dis. 2007;2(2):157-67. Int J Chron Obstruct Pulmon Dis. 2007. PMID: 18044688 Free PMC article.
-
Tiotropium bromide. A review of its use as maintenance therapy in patients with COPD.Treat Respir Med. 2004;3(4):247-68. doi: 10.2165/00151829-200403040-00005. Treat Respir Med. 2004. PMID: 15350163 Review.
-
Tiotropium versus placebo for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2012 Jul 11;(7):CD009285. doi: 10.1002/14651858.CD009285.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Jul 21;(7):CD009285. doi: 10.1002/14651858.CD009285.pub3. PMID: 22786525 Updated. Review.
Cited by
-
Comorbidities of patients in tiotropium clinical trials: comparison with observational studies of patients with chronic obstructive pulmonary disease.Int J Chron Obstruct Pulmon Dis. 2015 Mar 16;10:549-64. doi: 10.2147/COPD.S71913. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 25834416 Free PMC article. Review.
-
Health-related quality of life and chronic obstructive pulmonary disease in North Carolina.N Am J Med Sci. 2010 Feb;2(2):60-5. doi: 10.4297/najms.2010.260. N Am J Med Sci. 2010. PMID: 22624116 Free PMC article.
-
Optimizing bronchodilation in the prevention of COPD exacerbations.Respir Res. 2017 Jun 20;18(1):125. doi: 10.1186/s12931-017-0601-2. Respir Res. 2017. PMID: 28633665 Free PMC article. Review.
-
Clinical trial design in chronic obstructive pulmonary disease: current perspectives and considerations with regard to blinding of tiotropium.Respir Res. 2012 Jun 22;13(1):52. doi: 10.1186/1465-9921-13-52. Respir Res. 2012. PMID: 22726538 Free PMC article.
-
Emerging Therapeutic Options for the Management of COPD.Clin Med Insights Circ Respir Pulm Med. 2013 Apr 9;7:7-15. doi: 10.4137/CCRPM.S8140. Print 2013. Clin Med Insights Circ Respir Pulm Med. 2013. PMID: 23641160 Free PMC article.
References
-
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152(5 Pt 2):S77–121. - PubMed
-
- Bourbeau J, Maltais F, Rouleau M, et al. French-Canadian version of the Chronic Respiratory and St George’s Respiratory questionnaires: an assessment of their psychometric properties in patients with chronic obstructive pulmonary disease. Can Respir J. 2004;11:480–6. - PubMed
-
- Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217–24. - PubMed
-
- Celli B, ZuWallack R, Wang S, et al. Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumes. Chest. 2003;124:1743–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

