Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers
- PMID: 18685019
- PMCID: PMC3844752
- DOI: 10.1523/JNEUROSCI.1259-08.2008
Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers
Abstract
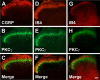
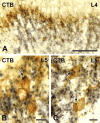
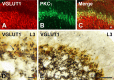
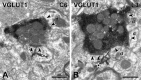
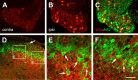
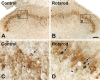
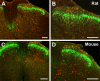
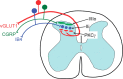
Protein kinase C gamma (PKCgamma), which is concentrated in interneurons of the inner part of lamina II of the dorsal horn, has been implicated in injury-induced allodynia, a condition wherein pain is produced by innocuous stimuli. Although it is generally assumed that these interneurons receive input from the nonpeptidergic, IB4-positive subset of nociceptors, the fact that PKCgamma cells do not express Fos in response to noxious stimulation suggests otherwise. Here, we demonstrate that the terminal field of the nonpeptidergic population of nociceptors, in fact, lies dorsal to that of PKCgamma interneurons. There was also no overlap between the PKCgamma-expressing interneurons and the transganglionic tracer wheat germ agglutinin which, after sciatic nerve injection, labels all unmyelinated nociceptors. However, transganglionic transport of the beta-subunit of cholera toxin, which marks the medium-diameter and large-diameter myelinated afferents that transmit non-noxious information, revealed extensive overlap with the layer of PKCgamma interneurons. Furthermore, expression of a transneuronal tracer in myelinated afferents resulted in labeling of PKCgamma interneurons. Light and electron microscopic double labeling further showed that the VGLUT1 subtype of vesicular glutamate transmitter, which is expressed in myelinated afferents, marks synapses that are presynaptic to the PKCgamma interneurons. Finally, we demonstrate that a continuous non-noxious input, generated by walking on a rotarod, induces Fos in the PKCgamma interneurons. These results establish that PKCgamma interneurons are activated by myelinated afferents that respond to innocuous stimuli, which suggests that injury-induced mechanical allodynia is transmitted through a circuit that involves PKCgamma interneurons and non-nociceptive, VGLUT1-expressing myelinated primary afferents.
Figures
Similar articles
-
Protein kinase C gamma interneurons in the rat medullary dorsal horn: distribution and synaptic inputs to these neurons, and subcellular localization of the enzyme.J Comp Neurol. 2014 Feb 1;522(2):393-413. doi: 10.1002/cne.23407. J Comp Neurol. 2014. PMID: 23818225
-
Triggering genetically-expressed transneuronal tracers by peripheral axotomy reveals convergent and segregated sensory neuron-spinal cord connectivity.Neuroscience. 2009 Nov 10;163(4):1220-32. doi: 10.1016/j.neuroscience.2009.07.051. Epub 2009 Jul 30. Neuroscience. 2009. PMID: 19647044 Free PMC article.
-
Contribution of dorsal horn CGRP-expressing interneurons to mechanical sensitivity.Elife. 2021 Jun 1;10:e59751. doi: 10.7554/eLife.59751. Elife. 2021. PMID: 34061020 Free PMC article.
-
PKCγ interneurons, a gateway to pathological pain in the dorsal horn.J Neural Transm (Vienna). 2020 Apr;127(4):527-540. doi: 10.1007/s00702-020-02162-6. Epub 2020 Feb 27. J Neural Transm (Vienna). 2020. PMID: 32108249 Review.
-
Transgenic Mouse Models for the Tracing of “Pain” Pathways.In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 7. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 7. PMID: 21882471 Free Books & Documents. Review.
Cited by
-
Intact subepidermal nerve fibers mediate mechanical hypersensitivity via the activation of protein kinase C gamma in spared nerve injury.Mol Pain. 2016 Jun 13;12:1744806916656189. doi: 10.1177/1744806916656189. Print 2016. Mol Pain. 2016. PMID: 27296621 Free PMC article.
-
The effects of preferential A- and C-fibre blocks and T-type calcium channel antagonist on detection of low-force monofilaments in healthy human participants.BMC Neurosci. 2015 Aug 13;16:52. doi: 10.1186/s12868-015-0190-2. BMC Neurosci. 2015. PMID: 26268809 Free PMC article.
-
Ionotropic glutamate receptors in spinal nociceptive processing.Mol Neurobiol. 2009 Dec;40(3):260-88. doi: 10.1007/s12035-009-8086-8. Epub 2009 Oct 31. Mol Neurobiol. 2009. PMID: 19876771 Review.
-
Hyperalgesic priming is restricted to isolectin B4-positive nociceptors.Neuroscience. 2010 Aug 11;169(1):431-5. doi: 10.1016/j.neuroscience.2010.04.082. Epub 2010 May 10. Neuroscience. 2010. PMID: 20457222 Free PMC article.
-
Nociceptive-induced myocardial remote conditioning is mediated by neuronal gamma protein kinase C.Basic Res Cardiol. 2013 Sep;108(5):381. doi: 10.1007/s00395-013-0381-x. Epub 2013 Aug 28. Basic Res Cardiol. 2013. PMID: 23982492 Free PMC article.
References
-
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–280. - PubMed
-
- Beal JA. Serial reconstruction of Ramon y Cajal's large primary afferent complexes in laminae II and III of the adult monkey spinal cord: a Golgi study. Brain Res. 1979;166:161–165. - PubMed
-
- Beal JA, Fox CA. Afferent fibers in the substantia gelatinosa of the adult monkey (Macaca mulatta): a Golgi study. J Comp Neurol. 1976;168:113–143. - PubMed
-
- Beal JA, Russell CT, Knight DS. Morphological and developmental characterization of local-circuit neurons in lamina III of the rat spinal cord. Neurosci Lett. 1988;86:1–5. - PubMed
-
- Bennett GJ, Abdelmoumene M, Hayashi H, Dubner R. Physiology and morphology of substantia gelatinosa neurons intracellularly stained with horseradish peroxidase. J Comp Neurol. 1980;194:809–827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

