Fusion of enhanced green fluorescent protein to the pseudorabies virus axonal sorting protein Us9 blocks anterograde spread of infection in mammalian neurons
- PMID: 18684822
- PMCID: PMC2566268
- DOI: 10.1128/JVI.01204-08
Fusion of enhanced green fluorescent protein to the pseudorabies virus axonal sorting protein Us9 blocks anterograde spread of infection in mammalian neurons
Abstract
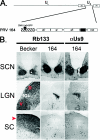
Pseudorabies virus encodes a membrane protein (Us9) that is essential for the axonal sorting of virus particles within neurons and anterograde spread in the mammalian nervous system. Enhanced green fluorescent protein (GFP)-tagged Us9 mimicked the trafficking properties of the wild-type protein in nonneuronal cells. We constructed a pseudorabies virus strain that expressed Us9-GFP and tested its spread capabilities in the rat visual system and in primary neuronal cultures. We report that Us9-EGFP does not promote anterograde spread of infection and may disrupt packing of viral membrane proteins in lipid rafts, an essential step for Us9-mediated axonal sorting.
Figures

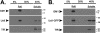
Similar articles
-
Visualization of an alphaherpesvirus membrane protein that is essential for anterograde axonal spread of infection in neurons.mBio. 2012 May 2;3(2):e00063-12. doi: 10.1128/mBio.00063-12. Print 2012. mBio. 2012. PMID: 22448044 Free PMC article.
-
Role of Us9 phosphorylation in axonal sorting and anterograde transport of pseudorabies virus.PLoS One. 2013;8(3):e58776. doi: 10.1371/journal.pone.0058776. Epub 2013 Mar 19. PLoS One. 2013. PMID: 23527020 Free PMC article.
-
Targeting of pseudorabies virus structural proteins to axons requires association of the viral Us9 protein with lipid rafts.PLoS Pathog. 2008 May 16;4(5):e1000065. doi: 10.1371/journal.ppat.1000065. PLoS Pathog. 2008. PMID: 18483549 Free PMC article.
-
The role of virion membrane protein endocytosis in the herpesvirus life cycle.J Clin Virol. 2000 Aug;17(2):69-82. doi: 10.1016/s1386-6532(00)00084-6. J Clin Virol. 2000. PMID: 10942087 Review.
-
Use of pseudorabies virus to delineate multisynaptic circuits in brain: opportunities and limitations.J Neurosci Methods. 2000 Nov 15;103(1):51-61. doi: 10.1016/s0165-0270(00)00295-8. J Neurosci Methods. 2000. PMID: 11074095 Review.
Cited by
-
Molecular features contributing to virus-independent intracellular localization and dynamic behavior of the herpesvirus transport protein US9.PLoS One. 2014 Aug 18;9(8):e104634. doi: 10.1371/journal.pone.0104634. eCollection 2014. PLoS One. 2014. PMID: 25133647 Free PMC article.
-
Proteomic Comparison of Three Wild-Type Pseudorabies Virus Strains and the Attenuated Bartha Strain Reveals Reduced Incorporation of Several Tegument Proteins in Bartha Virions.J Virol. 2022 Dec 21;96(24):e0115822. doi: 10.1128/jvi.01158-22. Epub 2022 Dec 1. J Virol. 2022. PMID: 36453884 Free PMC article.
-
Single-Particle Tracking of Virus Entry in Live Cells.Subcell Biochem. 2023;106:153-168. doi: 10.1007/978-3-031-40086-5_5. Subcell Biochem. 2023. PMID: 38159226 Review.
-
Comparison of the pseudorabies virus Us9 protein with homologs from other veterinary and human alphaherpesviruses.J Virol. 2009 Jul;83(14):6978-86. doi: 10.1128/JVI.00598-09. Epub 2009 May 6. J Virol. 2009. PMID: 19420087 Free PMC article.
-
The Attenuated Pseudorabies Virus Vaccine Strain Bartha Hyperactivates Plasmacytoid Dendritic Cells by Generating Large Amounts of Cell-Free Virus in Infected Epithelial Cells.J Virol. 2022 Jun 22;96(12):e0219921. doi: 10.1128/jvi.02199-21. Epub 2022 May 23. J Virol. 2022. PMID: 35604216 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

