Disruption of neuronal autophagy by infected microglia results in neurodegeneration
- PMID: 18682838
- PMCID: PMC2483417
- DOI: 10.1371/journal.pone.0002906
Disruption of neuronal autophagy by infected microglia results in neurodegeneration
Abstract
There is compelling evidence to support the idea that autophagy has a protective function in neurons and its disruption results in neurodegenerative disorders. Neuronal damage is well-documented in the brains of HIV-infected individuals, and evidence of inflammation, oxidative stress, damage to synaptic and dendritic structures, and neuronal loss are present in the brains of those with HIV-associated dementia. We investigated the role of autophagy in microglia-induced neurotoxicity in primary rodent neurons, primate and human models. We demonstrate here that products of simian immunodeficiency virus (SIV)-infected microglia inhibit neuronal autophagy, resulting in decreased neuronal survival. Quantitative analysis of autophagy vacuole numbers in rat primary neurons revealed a striking loss from the processes. Assessment of multiple biochemical markers of autophagic activity confirmed the inhibition of autophagy in neurons. Importantly, autophagy could be induced in neurons through rapamycin treatment, and such treatment conferred significant protection to neurons. Two major mediators of HIV-induced neurotoxicity, tumor necrosis factor-alpha and glutamate, had similar effects on reducing autophagy in neurons. The mRNA level of p62 was increased in the brain in SIV encephalitis and as well as in brains from individuals with HIV dementia, and abnormal neuronal p62 dot structures immunoreactivity was present and had a similar pattern with abnormal ubiquitinylated proteins. Taken together, these results identify that induction of deficits in autophagy is a significant mechanism for neurodegenerative processes that arise from glial, as opposed to neuronal, sources, and that the maintenance of autophagy may have a pivotal role in neuroprotection in the setting of HIV infection.
Conflict of interest statement
Figures
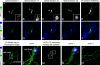
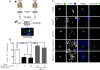
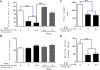

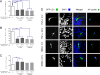

Similar articles
-
Decreased neuronal autophagy in HIV dementia: a mechanism of indirect neurotoxicity.Autophagy. 2008 Oct;4(7):963-6. doi: 10.4161/auto.6805. Epub 2008 Oct 18. Autophagy. 2008. PMID: 18772620 Free PMC article.
-
Neurovirulent simian immunodeficiency virus infection induces neuronal, endothelial, and glial apoptosis.Mol Med. 1996 Jul;2(4):417-28. Mol Med. 1996. PMID: 8827712 Free PMC article.
-
Disruption of excitatory amino acid transporters in brains of SIV-infected rhesus macaques is associated with microglia activation.J Neurochem. 2008 Jan;104(1):202-9. doi: 10.1111/j.1471-4159.2007.05007.x. Epub 2007 Nov 6. J Neurochem. 2008. PMID: 17986224
-
Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies.J Neurovirol. 2008 Aug;14(4):318-26. doi: 10.1080/13550280802132857. J Neurovirol. 2008. PMID: 18780233 Free PMC article. Review.
-
[The immunological manifestations and cytological characteristics of infection caused by the simian immunodeficiency virus (SIV) in rhesus monkeys].Tsitol Genet. 1993 Nov-Dec;27(6):97-104. Tsitol Genet. 1993. PMID: 8066812 Review. Russian.
Cited by
-
Electro-Magnetic Nano-Particle Bound Beclin1 siRNA Crosses the Blood-Brain Barrier to Attenuate the Inflammatory Effects of HIV-1 Infection in Vitro.J Neuroimmune Pharmacol. 2017 Mar;12(1):120-132. doi: 10.1007/s11481-016-9688-3. Epub 2016 Jun 10. J Neuroimmune Pharmacol. 2017. PMID: 27287620 Free PMC article.
-
Pathogenesis of age-related HIV neurodegeneration.J Neurovirol. 2019 Oct;25(5):622-633. doi: 10.1007/s13365-019-00728-z. Epub 2019 Feb 21. J Neurovirol. 2019. PMID: 30790184 Free PMC article. Review.
-
Elevated ATG5 expression in autoimmune demyelination and multiple sclerosis.Autophagy. 2009 Feb;5(2):152-8. doi: 10.4161/auto.5.2.7348. Epub 2009 Feb 5. Autophagy. 2009. PMID: 19066443 Free PMC article.
-
Autophagy, inflammation and neurodegenerative disease.Eur J Neurosci. 2011 Jan;33(2):197-204. doi: 10.1111/j.1460-9568.2010.07500.x. Epub 2010 Dec 7. Eur J Neurosci. 2011. PMID: 21138487 Free PMC article. Review.
-
Role of neurotrophic factor alterations in the neurodegenerative process in HIV associated neurocognitive disorders.J Neuroimmune Pharmacol. 2014 Mar;9(2):102-16. doi: 10.1007/s11481-013-9520-2. Epub 2014 Feb 8. J Neuroimmune Pharmacol. 2014. PMID: 24510686 Free PMC article. Review.
References
-
- Komatsu M, Ueno T, Waguri S, Uchiyama Y, Kominami E, et al. Constitutive autophagy: vital role in clearance of unfavorable proteins in neurons. Cell Death Differ. 2007;14:887–894. - PubMed
-
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. - PubMed
-
- Boland B, Nixon RA. Neuronal macroautophagy: from development to degeneration. Mol Aspects Med. 2006;27:503–519. - PubMed
-
- Rideout HJ, Lang-Rollin I, Stefanis L. Involvement of macroautophagy in the dissolution of neuronal inclusions. Int J Biochem Cell Biol. 2004;36:2551–2562. - PubMed
-
- Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–312. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

