Re-evaluating the roles of proposed modulators of mammalian target of rapamycin complex 1 (mTORC1) signaling
- PMID: 18676370
- PMCID: PMC2662142
- DOI: 10.1074/jbc.M803348200
Re-evaluating the roles of proposed modulators of mammalian target of rapamycin complex 1 (mTORC1) signaling
Abstract
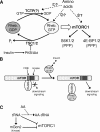
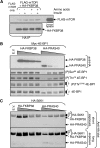
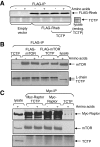
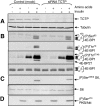
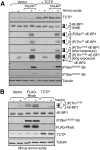
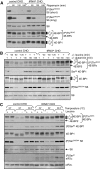
Signaling through mammalian target of rapamycin complex 1 (mTORC1) is stimulated by amino acids and insulin. Insulin inactivates TSC1/2, the GTPase-activator complex for Rheb, and Rheb.GTP activates mTORC1. It is not clear how amino acids regulate mTORC1. FKBP38 (immunophilin FK506-binding protein, 38 kDa), was recently reported to exert a negative effect on mTORC1 function that is relieved by its binding to Rheb.GTP. We confirm that Rheb binds wild type FKBP38, but inactive Rheb mutants showed contrasting abilities to bind FKBP38. We were unable to observe any regulation of FKBP38/mTOR binding by amino acids or insulin. Furthermore, FKBP38 did not inhibit mTORC1 signaling. The translationally controlled tumor protein (TCTP) in Drosophila was recently reported to act as the guanine nucleotide-exchange factor for Rheb. We have studied the role of TCTP in mammalian TORC1 signaling and its control by amino acids. Reducing TCTP levels did not reproducibly affect mTORC1 signaling in amino acid-replete/insulin-stimulated cells. Moreover, overexpressing TCTP did not rescue mTORC1 signaling in amino acid-starved cells. In addition, we were unable to see any stable interaction between TCTP and Rheb or mTORC1. Accumulation of uncharged tRNA has been previously proposed to be involved in the inhibition of mTORC1 signaling during amino acid starvation. To test this hypothesis, we used a Chinese hamster ovary cell line containing a temperature-sensitive mutation in leucyl-tRNA synthetase. Leucine deprivation markedly inhibited mTORC1 signaling in these cells, but shifting the cells to the nonpermissive temperature for the synthetase did not. These data indicate that uncharged tRNA(Leu) does not switch off mTORC1 signaling and suggest that mTORC1 is controlled by a distinct pathway that senses the availability of amino acids. Our data also indicate that, in the mammalian cell lines tested here, neither TCTP nor FKBP38 regulates mTORC1 signaling.
Figures

Similar articles
-
Amino acid regulation of TOR complex 1.Am J Physiol Endocrinol Metab. 2009 Apr;296(4):E592-602. doi: 10.1152/ajpendo.90645.2008. Epub 2008 Sep 2. Am J Physiol Endocrinol Metab. 2009. PMID: 18765678 Free PMC article. Review.
-
Specific activation of mTORC1 by Rheb G-protein in vitro involves enhanced recruitment of its substrate protein.J Biol Chem. 2009 May 8;284(19):12783-91. doi: 10.1074/jbc.M809207200. Epub 2009 Mar 19. J Biol Chem. 2009. PMID: 19299511 Free PMC article.
-
RhoA modulates signaling through the mechanistic target of rapamycin complex 1 (mTORC1) in mammalian cells.Cell Signal. 2014 Mar;26(3):461-7. doi: 10.1016/j.cellsig.2013.11.035. Epub 2013 Dec 3. Cell Signal. 2014. PMID: 24316235 Free PMC article.
-
Control of TSC2-Rheb signaling axis by arginine regulates mTORC1 activity.Elife. 2016 Jan 7;5:e11058. doi: 10.7554/eLife.11058. Elife. 2016. PMID: 26742086 Free PMC article.
-
To cease or to proliferate: new insights into TCTP function from a Drosophila study.Cell Adh Migr. 2007 Jul-Sep;1(3):129-30. doi: 10.4161/cam.1.3.4901. Epub 2007 Jul 16. Cell Adh Migr. 2007. PMID: 19262129 Free PMC article. Review.
Cited by
-
Neighborhood properties are important determinants of temperature sensitive mutations.PLoS One. 2011;6(12):e28507. doi: 10.1371/journal.pone.0028507. Epub 2011 Dec 2. PLoS One. 2011. PMID: 22164302 Free PMC article.
-
Phosphatidic acid signaling to mTOR: signals for the survival of human cancer cells.Biochim Biophys Acta. 2009 Sep;1791(9):949-55. doi: 10.1016/j.bbalip.2009.02.009. Epub 2009 Mar 2. Biochim Biophys Acta. 2009. PMID: 19264150 Free PMC article. Review.
-
Fortilin: A Potential Target for the Prevention and Treatment of Human Diseases.Adv Clin Chem. 2017;82:265-300. doi: 10.1016/bs.acc.2017.06.006. Epub 2017 Aug 7. Adv Clin Chem. 2017. PMID: 28939212 Free PMC article. Review.
-
Regulation of TOR by small GTPases.EMBO Rep. 2012 Feb 1;13(2):121-8. doi: 10.1038/embor.2011.257. EMBO Rep. 2012. PMID: 22240970 Free PMC article. Review.
-
Dynamic switch of negative feedback regulation in Drosophila Akt-TOR signaling.PLoS Genet. 2010 Jun 17;6(6):e1000990. doi: 10.1371/journal.pgen.1000990. PLoS Genet. 2010. PMID: 20585550 Free PMC article.
References
-
- Wullschleger, S., Loewith, R., and Hall, M. N. (2006) Cell 124 471–484 - PubMed
-
- Yang, Q., and Guan, K. L. (2007) Cell Res. 17 666–681 - PubMed
-
- Easton, J. B., and Houghton, P. J. (2006) Oncogene 25 6436–6446 - PubMed
-
- Lee, C. H., Inoki, K., and Guan, K. L. (2007) Annu. Rev. Pharmacol. Toxicol. 47 443–467 - PubMed
-
- Wang, X., and Proud, C. G. (2006) Physiol. (Bethesda) 21 362–369 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

