A hyperfusogenic F protein enhances the oncolytic potency of a paramyxovirus simian virus 5 P/V mutant without compromising sensitivity to type I interferon
- PMID: 18667520
- PMCID: PMC2546974
- DOI: 10.1128/JVI.01054-08
A hyperfusogenic F protein enhances the oncolytic potency of a paramyxovirus simian virus 5 P/V mutant without compromising sensitivity to type I interferon
Abstract
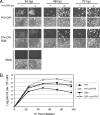
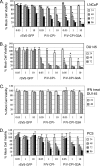
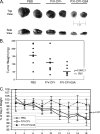
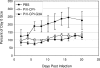
Viral fusogenic membrane proteins have been proposed as tools to increase the potency of oncolytic viruses, but there is a need for mechanisms to control the spread of fusogenic viruses in normal versus tumor cells. We have previously shown that a mutant of the paramyxovirus simian virus 5 (SV5) that harbors mutations in the P/V gene from the canine parainfluenza virus (P/V-CPI(-)) is a potent inducer of type I interferon (IFN) and apoptosis and is restricted for spread through normal but not tumor cells in vitro. Here, we have used the cytopathic P/V-CPI(-) as a backbone vector to test the hypothesis that a virus expressing a hyperfusogenic glycoprotein will be a more effective oncolytic vector but will retain sensitivity to IFN. A P/V mutant virus expressing an F protein with a glycine-to-alanine substitution in the fusion peptide (P/V-CPI(-)-G3A) was more fusogenic than the parental P/V-CPI(-) mutant. In two model prostate tumor cell lines which are defective in IFN production (LNCaP and DU145), the hyperfusogenic P/V-CPI(-)-G3A mutant had normal growth properties at low multiplicities of infection and was more effective than the parental P/V-CPI(-) mutant at cell killing in vitro. However, in PC3 cells which produce and respond to IFN, the hyperfusogenic P/V-CPI(-)-G3A mutant was attenuated for growth and spread. Killing of PC3 cells was equivalent between the parental P/V-CPI(-) mutant and the hyperfusogenic P/V-CPI(-)-G3A mutant. In a nude mouse model using LNCaP cells, the hyperfusogenic P/V-CPI(-)-G3A mutant was more effective than P/V-CPI(-) at reducing tumor burden. In the case of DU145 tumors, the two vectors based on P/V-CPI(-) were equally effective at limiting tumor growth. Together, our results provide proof of principle that a cytopathic SV5 P/V mutant can serve as an oncolytic virus and that the oncolytic effectiveness of P/V mutants can be enhanced by a fusogenic membrane protein without compromising sensitivity to IFN. The potential advantages of SV5-based oncolytic vectors are discussed.
Figures
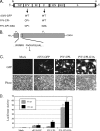
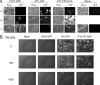
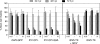
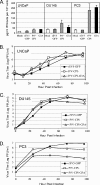
Similar articles
-
A simian virus 5 (SV5) P/V mutant is less cytopathic than wild-type SV5 in human dendritic cells and is a more effective activator of dendritic cell maturation and function.J Virol. 2006 Apr;80(7):3416-27. doi: 10.1128/JVI.80.7.3416-3427.2006. J Virol. 2006. PMID: 16537609 Free PMC article.
-
Growth sensitivity of a recombinant simian virus 5 P/V mutant to type I interferon differs between tumor cell lines and normal primary cells.Virology. 2005 Apr 25;335(1):131-44. doi: 10.1016/j.virol.2005.02.004. Virology. 2005. PMID: 15823612
-
Naturally occurring substitutions in the P/V gene convert the noncytopathic paramyxovirus simian virus 5 into a virus that induces alpha/beta interferon synthesis and cell death.J Virol. 2002 Oct;76(20):10109-21. doi: 10.1128/jvi.76.20.10109-10121.2002. J Virol. 2002. PMID: 12239285 Free PMC article.
-
Apoptosis induction and interferon signaling but not IFN-beta promoter induction by an SV5 P/V mutant are rescued by coinfection with wild-type SV5.Virology. 2003 Nov 10;316(1):41-54. doi: 10.1016/s0042-6822(03)00584-1. Virology. 2003. PMID: 14599789
-
Molecular biology of canine parainfluenza virus V protein and its potential applications in tumor immunotherapy.Front Microbiol. 2023 Dec 20;14:1282112. doi: 10.3389/fmicb.2023.1282112. eCollection 2023. Front Microbiol. 2023. PMID: 38173672 Free PMC article. Review.
Cited by
-
Differential In Vitro Growth and Cell Killing of Cancer versus Benign Prostate Cells by Oncolytic Parainfluenza Virus.Pathogens. 2022 Apr 21;11(5):493. doi: 10.3390/pathogens11050493. Pathogens. 2022. PMID: 35631014 Free PMC article.
-
LaSota fusion (F) cleavage motif-mediated fusion activity is affected by other regions of the F protein from different genotype Newcastle disease virus in a chimeric virus: implication for virulence attenuation.J Gen Virol. 2016 Jun;97(6):1297-1303. doi: 10.1099/jgv.0.000439. Epub 2016 Mar 1. J Gen Virol. 2016. Retraction in: J Gen Virol. 2020 Sep;101(9):1019. doi: 10.1099/jgv.0.001463 PMID: 26932300 Free PMC article. Retracted.
-
Oncolysis by paramyxoviruses: multiple mechanisms contribute to therapeutic efficiency.Mol Ther Oncolytics. 2015;2:15011-. doi: 10.1038/mto.2015.11. Epub 2015 Jul 22. Mol Ther Oncolytics. 2015. PMID: 26640816 Free PMC article.
-
Current good manufacturing practice production of an oncolytic recombinant vesicular stomatitis viral vector for cancer treatment.Hum Gene Ther. 2011 Apr;22(4):489-97. doi: 10.1089/hum.2010.159. Epub 2011 Mar 8. Hum Gene Ther. 2011. PMID: 21083425 Free PMC article.
-
Measles virus mutants possessing the fusion protein with enhanced fusion activity spread effectively in neuronal cells, but not in other cells, without causing strong cytopathology.J Virol. 2015 Mar;89(5):2710-7. doi: 10.1128/JVI.03346-14. Epub 2014 Dec 17. J Virol. 2015. PMID: 25520515 Free PMC article.
References
-
- Ahmed, M., S. D. Cramer, and D. S. Lyles. 2004. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology 33034-49. - PubMed
-
- Bateman, A., F. Bullough, S. Murphy, L. Emiliusen, D. Lavillette, F. L. Cosset, R. Cattaneo, S. J. Russell, and R. G. Vile. 2000. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res. 601492-1497. - PubMed
-
- Bateman, A. R., K. J. Harrington, T. Kottke, A. Ahmed, A. A. Melcher, M. J. Gough, E. Linardakis, D. Riddle, A. Dietz, C. M. Lohse, S. Strome, T. Peterson, R. Simari, and R. G. Vile. 2002. Viral fusogenic membrane glycoproteins kill solid tumor cells by nonapoptotic mechanisms that promote cross presentation of tumor antigens by dendritic cells. Cancer Res. 626566-6578. - PubMed
-
- Bell, J. C., B. Lichty, and D. Stojdl. 2003. Getting oncolytic virus therapies off the ground. Cancer Cell 47-11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

