Localization of synucleins in the mammalian cochlea
- PMID: 18665422
- PMCID: PMC2580813
- DOI: 10.1007/s10162-008-0134-y
Localization of synucleins in the mammalian cochlea
Abstract
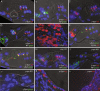
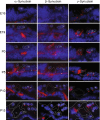
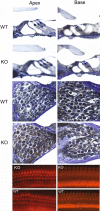
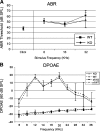
Synucleins are widely expressed synaptic proteins within the central nervous system that have been implicated in such neurodegenerative disorders as Parkinson's disease. In this study, an initial characterization of all three synucleins, alpha-, beta-, and gamma-synuclein, within the cochlea was undertaken. Reverse transcriptase-polymerase chain reaction (PCR) demonstrated all three synuclein mRNA species within microdissected cochlear tissue. Quantitative PCR suggests that beta-synuclein is the most abundantly expressed form, followed by gamma- and then alpha-synuclein. Western blot analysis similarly demonstrates all three synuclein proteins within microdissected cochlear tissue. Immunofluorescence localizes the three synucleins predominantly to the efferent neuronal system at the efferent outer hair cell synapse, with some additional localization within the efferent tunnel-crossing fibers (alpha- and gamma-synuclein), spiral ganglion (beta-synuclein), inner spiral bundle (gamma-synuclein), and stria vascularis (alpha- > beta-synuclein). Developmentally, gamma-synuclein can be seen in the region of the outer hair cells by E19, while alpha- and beta-synuclein do not clearly appear there until approximately P10. Additional studies in a null-mutant gamma-synuclein mouse show no histological changes in the organ of Corti with normal hair cell and spiral ganglion cell counts, and normal ABR and DPOAE thresholds in wild-type vs mutant littermates. Together, these results localize synucleins to the efferent cholinergic neuronal auditory system, pointing to a role in normal auditory function, and raising the potential implications for their role in auditory neurodegenerative disorders. However, gamma-synuclein alone is not required for the development and maintenance of normal hearing through P21. Whether overlapping roles of the other synucleins help compensate for the loss of gamma-synuclein remains to be determined.
Figures


Similar articles
-
Muscarinic signaling in the cochlea: presynaptic and postsynaptic effects on efferent feedback and afferent excitability.J Neurosci. 2010 May 12;30(19):6751-62. doi: 10.1523/JNEUROSCI.5080-09.2010. J Neurosci. 2010. PMID: 20463237 Free PMC article.
-
Exploring Intrinsic Disorder in Human Synucleins and Associated Proteins.Int J Mol Sci. 2024 Aug 1;25(15):8399. doi: 10.3390/ijms25158399. Int J Mol Sci. 2024. PMID: 39125972 Free PMC article.
-
Dopaminergic signaling in the cochlea: receptor expression patterns and deletion phenotypes.J Neurosci. 2012 Jan 4;32(1):344-55. doi: 10.1523/JNEUROSCI.4720-11.2012. J Neurosci. 2012. PMID: 22219295 Free PMC article.
-
Nicotinic acetylcholine receptor structure and function in the efferent auditory system.Anat Rec A Discov Mol Cell Evol Biol. 2006 Apr;288(4):424-34. doi: 10.1002/ar.a.20302. Anat Rec A Discov Mol Cell Evol Biol. 2006. PMID: 16550589 Review.
-
Fish Synucleins: An Update.Mar Drugs. 2015 Oct 30;13(11):6665-86. doi: 10.3390/md13116665. Mar Drugs. 2015. PMID: 26528989 Free PMC article. Review.
Cited by
-
Relationship between Hearing Loss and Dementia Differs According to the Underlying Mechanism.J Clin Neurol. 2021 Apr;17(2):290-299. doi: 10.3988/jcn.2021.17.2.290. J Clin Neurol. 2021. PMID: 33835751 Free PMC article.
-
Recombinant α- β- and γ-Synucleins Stimulate Protein Phosphatase 2A Catalytic Subunit Activity in Cell Free Assays.J Vis Exp. 2017 Aug 13;(126):55361. doi: 10.3791/55361. J Vis Exp. 2017. PMID: 28829427 Free PMC article.
-
Future Perspectives on the Relevance of Auditory Markers in Prodromal Parkinson's Disease.Front Neurol. 2020 Jul 16;11:689. doi: 10.3389/fneur.2020.00689. eCollection 2020. Front Neurol. 2020. PMID: 32765404 Free PMC article. Review.
-
Hearing Loss in Neurological Disorders.Front Cell Dev Biol. 2021 Aug 11;9:716300. doi: 10.3389/fcell.2021.716300. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34458270 Free PMC article. Review.
-
Relationship of Hearing Loss to Parkinson's Disease, Dementia, and APOE Genotype in Adults.Medicina (Kaunas). 2024 Apr 25;60(5):703. doi: 10.3390/medicina60050703. Medicina (Kaunas). 2024. PMID: 38792885 Free PMC article.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0896-6273(00)80886-7', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0896-6273(00)80886-7'}, {'type': 'PubMed', 'value': '10707987', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10707987/'}]}
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 25:239–252, 2000. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1097/WNR.0b013e3280115185', 'is_inner': False, 'url': 'https://doi.org/10.1097/wnr.0b013e3280115185'}, {'type': 'PubMed', 'value': '17179863', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17179863/'}]}
- Adamczyk A, Strosznajder JB. Alpha-synuclein potentiates Ca2+ influx through voltage-dependent Ca2+ channels. Neuroreport. 17:1883–1886, 2006. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1523/JNEUROSCI.3746-06.2006', 'is_inner': False, 'url': 'https://doi.org/10.1523/jneurosci.3746-06.2006'}, {'type': 'PMC', 'value': 'PMC6674959', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC6674959/'}, {'type': 'PubMed', 'value': '17167097', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17167097/'}]}
- Akil O, Chang J, Hiel H, Kong JH, Yi E, Glowatzki E, Lustig LR. Progressive deafness and altered cochlear innervation in knock-out mice lacking prosaposin. J. Neurosci. 26:13076–13088, 2006. - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/cne.903060304', 'is_inner': False, 'url': 'https://doi.org/10.1002/cne.903060304'}, {'type': 'PubMed', 'value': '1865000', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/1865000/'}]}
- Berglund AM, Ryugo DK. Neurofilament antibodies and spiral ganglion neurons of the mammalian cochlea. J. Comp. Neurol. 306:393–408, 1991. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1007/s10048-008-0124-6', 'is_inner': False, 'url': 'https://doi.org/10.1007/s10048-008-0124-6'}, {'type': 'PubMed', 'value': '18335262', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/18335262/'}]}
- Beyer K, Domingo-Sabat M, Humbert J, Carrato C, Ferrer I, Ariza A. Differential expression of alpha-synuclein, parkin, and synphilin-1 isoforms in Lewy body disease. Neurogenetics 9:163–172, 2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

