Quality control of photosystem II: reactive oxygen species are responsible for the damage to photosystem II under moderate heat stress
- PMID: 18664569
- PMCID: PMC2661399
- DOI: 10.1074/jbc.M710465200
Quality control of photosystem II: reactive oxygen species are responsible for the damage to photosystem II under moderate heat stress
Abstract
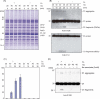
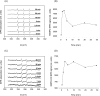
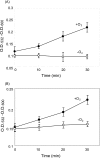
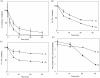
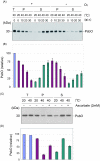
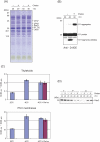
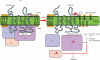
Moderate heat stress (40 degrees C for 30 min) on spinach thylakoid membranes induced cleavage of the reaction center-binding D1 protein of photosystem II, aggregation of the D1 protein with the neighboring polypeptides D2 and CP43, and release of three extrinsic proteins, PsbO, -P, and -Q. These heat-induced events were suppressed under anaerobic conditions or by the addition of sodium ascorbate, a general scavenger of reactive oxygen species. In accordance with this, singlet oxygen and hydroxyl radicals were detected in spinach photosystem II membranes incubated at 40 degrees C for 30 min with electron paramagnetic resonance spin-trapping spectroscopy. The moderate heat stress also induced significant lipid peroxidation under aerobic conditions. We suggest that the reactive oxygen species are generated by heat-induced inactivation of a water-oxidizing manganese complex and through lipid peroxidation. Although occurring in the dark, the damages caused by the moderate heat stress to photosystem II are quite similar to those induced by excessive illumination where reactive oxygen species are involved.
Figures



Similar articles
-
Quality control of photosystem II: lipid peroxidation accelerates photoinhibition under excessive illumination.PLoS One. 2012;7(12):e52100. doi: 10.1371/journal.pone.0052100. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300595 Free PMC article.
-
Quality control of photosystem II: Thylakoid unstacking is necessary to avoid further damage to the D1 protein and to facilitate D1 degradation under light stress in spinach thylakoids.J Biol Chem. 2009 Sep 11;284(37):25343-52. doi: 10.1074/jbc.M109.007740. Epub 2009 Jul 17. J Biol Chem. 2009. PMID: 19617353 Free PMC article.
-
Quality control of Photosystem II: cleavage and aggregation of heat-damaged D1 protein in spinach thylakoids.Biochim Biophys Acta. 2007 Jun;1767(6):838-46. doi: 10.1016/j.bbabio.2007.05.001. Epub 2007 May 10. Biochim Biophys Acta. 2007. PMID: 17543883
-
Damage to photosystem II by lipid peroxidation products.Biochim Biophys Acta Gen Subj. 2017 Feb;1861(2):457-466. doi: 10.1016/j.bbagen.2016.10.005. Epub 2016 Oct 11. Biochim Biophys Acta Gen Subj. 2017. PMID: 27741410 Review.
-
Quality control of photosystem II: impact of light and heat stresses.Photosynth Res. 2008 Oct-Dec;98(1-3):589-608. doi: 10.1007/s11120-008-9372-4. Epub 2008 Oct 21. Photosynth Res. 2008. PMID: 18937045 Review.
Cited by
-
Quality control of PSII: behavior of PSII in the highly crowded grana thylakoids under excessive light.Plant Cell Physiol. 2014 Jul;55(7):1206-15. doi: 10.1093/pcp/pcu043. Epub 2014 Mar 7. Plant Cell Physiol. 2014. PMID: 24610582 Free PMC article. Review.
-
Quality Control of Photosystem II: The Mechanisms for Avoidance and Tolerance of Light and Heat Stresses are Closely Linked to Membrane Fluidity of the Thylakoids.Front Plant Sci. 2016 Aug 2;7:1136. doi: 10.3389/fpls.2016.01136. eCollection 2016. Front Plant Sci. 2016. PMID: 27532009 Free PMC article. Review.
-
Identification of components associated with thermal acclimation of photosystem II in Synechocystis sp. PCC6803.PLoS One. 2010 May 6;5(5):e10511. doi: 10.1371/journal.pone.0010511. PLoS One. 2010. PMID: 20463904 Free PMC article.
-
Antisense reductions in the PsbO protein of photosystem II leads to decreased quantum yield but similar maximal photosynthetic rates.J Exp Bot. 2012 Aug;63(13):4781-95. doi: 10.1093/jxb/ers156. J Exp Bot. 2012. PMID: 22922640 Free PMC article.
-
The physiological role of ascorbate as photosystem II electron donor: protection against photoinactivation in heat-stressed leaves.Plant Physiol. 2011 May;156(1):382-92. doi: 10.1104/pp.110.171918. Epub 2011 Feb 28. Plant Physiol. 2011. PMID: 21357184 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

