Lysosomes are the major vesicular compartment undergoing Ca2+-regulated exocytosis from cortical astrocytes
- PMID: 18650341
- PMCID: PMC6670856
- DOI: 10.1523/JNEUROSCI.0744-08.2008
Lysosomes are the major vesicular compartment undergoing Ca2+-regulated exocytosis from cortical astrocytes
Abstract
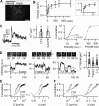
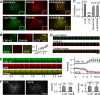
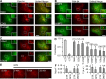
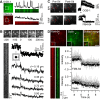
Although Ca(2+)-dependent exocytosis is considered to be a pathway for gliotransmitter release from astrocytes, the structural and functional bases of this process remain controversial. We studied the relationship between near-membrane Ca(2+) elevations and the dynamics of single astroglial vesicles with styryl (FM) dyes. We show that cultured astrocytes, unlike neurons, spontaneously internalize FM dyes, resulting in the labeling of the entire acidic vesicle population within minutes. Interestingly, metabotropic glutamate receptor activation did not affect the FM labeling. Most FM-stained vesicles expressed sialin, CD63/LAMP3, and VAMP7, three markers for lysosomes and late endosomes. A subset of lysosomes underwent asynchronous exocytosis that required both Ca(2+) mobilization from intracellular stores and Ca(2+) influx across the plasma membrane. Lysosomal fusion occurred within seconds and was complete with no evidence for kiss and run. Our experiments suggest that astroglial Ca(2+)-regulated exocytosis is carried by lysosomes and operates on a timescale orders of magnitude slower than synaptic transmission.
Figures

Similar articles
-
SDF 1-alpha (CXCL12) triggers glutamate exocytosis from astrocytes on a millisecond time scale: imaging analysis at the single-vesicle level with TIRF microscopy.J Neuroimmunol. 2008 Jul 31;198(1-2):82-91. doi: 10.1016/j.jneuroim.2008.04.015. Epub 2008 Jun 6. J Neuroimmunol. 2008. PMID: 18538866
-
Calcium triggers exocytosis from two types of organelles in a single astrocyte.J Neurosci. 2011 Jul 20;31(29):10593-601. doi: 10.1523/JNEUROSCI.6401-10.2011. J Neurosci. 2011. PMID: 21775603 Free PMC article.
-
Ca2+ regulation of dynamin-independent endocytosis in cortical astrocytes.J Neurosci. 2009 Jun 24;29(25):8063-74. doi: 10.1523/JNEUROSCI.6139-08.2009. J Neurosci. 2009. PMID: 19553446 Free PMC article.
-
Loose excitation-secretion coupling in astrocytes.Glia. 2016 May;64(5):655-67. doi: 10.1002/glia.22920. Epub 2015 Sep 11. Glia. 2016. PMID: 26358496 Free PMC article. Review.
-
"Kiss-and-run" exocytosis in astrocytes.Neuroscientist. 2006 Oct;12(5):375-8. doi: 10.1177/1073858406291588. Neuroscientist. 2006. PMID: 16956999 Review.
Cited by
-
Astroglial S100B Secretion Is Mediated by Ca2+ Mobilization from Endoplasmic Reticulum: A Study Using Forskolin and DMSO as Secretagogues.Int J Mol Sci. 2023 Nov 21;24(23):16576. doi: 10.3390/ijms242316576. Int J Mol Sci. 2023. PMID: 38068900 Free PMC article.
-
Gliocrine System: Astroglia as Secretory Cells of the CNS.Adv Exp Med Biol. 2019;1175:93-115. doi: 10.1007/978-981-13-9913-8_4. Adv Exp Med Biol. 2019. PMID: 31583585 Free PMC article. Review.
-
Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior.Neuropharmacology. 2009 Sep;57(4):343-6. doi: 10.1016/j.neuropharm.2009.06.031. Epub 2009 Jul 3. Neuropharmacology. 2009. PMID: 19577581 Free PMC article. Review.
-
New tools for investigating astrocyte-to-neuron communication.Front Cell Neurosci. 2013 Oct 29;7:193. doi: 10.3389/fncel.2013.00193. Front Cell Neurosci. 2013. PMID: 24194698 Free PMC article. Review.
-
Novel astrocyte targets: new avenues for the therapeutic treatment of epilepsy.Neuroscientist. 2015 Feb;21(1):62-83. doi: 10.1177/1073858414523320. Epub 2014 Mar 7. Neuroscientist. 2015. PMID: 24609207 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

