Monkeypox virus viral chemokine inhibitor (MPV vCCI), a potent inhibitor of rhesus macrophage inflammatory protein-1
- PMID: 18639466
- PMCID: PMC2547134
- DOI: 10.1016/j.cyto.2008.05.016
Monkeypox virus viral chemokine inhibitor (MPV vCCI), a potent inhibitor of rhesus macrophage inflammatory protein-1
Abstract
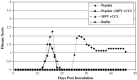
Monkeypox virus (MPV) is an orthopoxvirus with considerable homology to variola major, the etiologic agent of smallpox. Although smallpox was eradicated in 1976, the outbreak of MPV in the U.S. highlights the health hazards associated with zoonotic infections. Like other orthopoxviruses, MPV encodes a secreted chemokine binding protein, vCCI that is abundantly expressed and secreted from MPV infected cells. EMSA data shows vCCI efficiently binds rhesus MIP-1alpha (rhMIP-1alpha) at near one to one stoichiometry. In vitro chemotaxis experiments demonstrate that vCCI completely inhibits rhMIP-1alpha mediated chemotaxis, while in vivo recruitment assays in rhesus macaques using chemokine-saturated implants show a decrease in the number of CD14(+) cells responding to rhMIP-1alpha when vCCI is present, suggesting vCCI is effectively inhibiting chemokine function both in vitro and in vivo. More importantly, we demonstrate that vCCI can diminish the severity of the acute phase and completely inhibit the relapsing phase of experimental allergic encephalomyelitis (EAE) disease. These data represent the first in vitro and in vivo characterization of vCCI emphasizing its function as a potent inhibitor of rhMIP-1alpha. Furthermore, the ability of vCCI to inhibit relapsing EAE disease represents a novel therapeutic approach for treating chemokine-mediated diseases.
Figures

Similar articles
-
Solution structure of the complex between poxvirus-encoded CC chemokine inhibitor vCCI and human MIP-1beta.Proc Natl Acad Sci U S A. 2006 Sep 19;103(38):13985-90. doi: 10.1073/pnas.0602142103. Epub 2006 Sep 8. Proc Natl Acad Sci U S A. 2006. PMID: 16963564 Free PMC article.
-
Structural insights into the interaction between a potent anti-inflammatory protein, viral CC chemokine inhibitor (vCCI), and the human CC chemokine, Eotaxin-1.J Biol Chem. 2014 Mar 7;289(10):6592-6603. doi: 10.1074/jbc.M113.538991. Epub 2014 Jan 30. J Biol Chem. 2014. PMID: 24482230 Free PMC article.
-
Poxvirus genomes encode a secreted, soluble protein that preferentially inhibits beta chemokine activity yet lacks sequence homology to known chemokine receptors.Virology. 1997 Sep 29;236(2):316-27. doi: 10.1006/viro.1997.8730. Virology. 1997. PMID: 9325239
-
Variola virus immune evasion proteins.Microbes Infect. 2003 Sep;5(11):1049-56. doi: 10.1016/s1286-4579(03)00194-1. Microbes Infect. 2003. PMID: 12941397 Review.
-
The binding and specificity of chemokine binding proteins, through the lens of experiment and computation.Biomed J. 2022 Jun;45(3):439-453. doi: 10.1016/j.bj.2021.07.004. Epub 2021 Jul 24. Biomed J. 2022. PMID: 34311129 Free PMC article. Review.
Cited by
-
Exploring the key genomic variation in monkeypox virus during the 2022 outbreak.BMC Genom Data. 2023 Nov 16;24(1):67. doi: 10.1186/s12863-023-01171-0. BMC Genom Data. 2023. PMID: 37968621 Free PMC article.
-
Cytomegalovirus CC chemokine promotes immune cell migration.J Virol. 2012 Nov;86(21):11833-44. doi: 10.1128/JVI.00452-12. Epub 2012 Aug 22. J Virol. 2012. PMID: 22915808 Free PMC article.
-
A novel highly potent therapeutic antibody neutralizes multiple human chemokines and mimics viral immune modulation.PLoS One. 2012;7(8):e43332. doi: 10.1371/journal.pone.0043332. Epub 2012 Aug 17. PLoS One. 2012. PMID: 22912856 Free PMC article.
-
Systems integration of biodefense omics data for analysis of pathogen-host interactions and identification of potential targets.PLoS One. 2009 Sep 25;4(9):e7162. doi: 10.1371/journal.pone.0007162. PLoS One. 2009. PMID: 19779614 Free PMC article.
-
Structural Conservation and Functional Diversity of the Poxvirus Immune Evasion (PIE) Domain Superfamily.Viruses. 2015 Aug 28;7(9):4878-98. doi: 10.3390/v7092848. Viruses. 2015. PMID: 26343707 Free PMC article. Review.
References
-
- Shchelkunov S.N., et al. Multiple genetic differences between the monkeypox and variola viruses. Dokl Biochem Biophys. 2002;384:143–147. - PubMed
-
- Control C.f.D. Questions and Answers About Monkeypox. 2003.
-
- Prevention C.f.D.C.a. Questions and Answers About Monkeypox. 2003.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

